Lewis acid catalyzed diastereoselective [3+4]-annulation of donor–acceptor cyclopropanes with anthranils: synthesis of tetrahydro-1-benzazepine derivatives†
Zhe-Hao
Wang
,
Huan-Huan
Zhang
*,
Dao-Ming
Wang
,
Peng-Fei
Xu
 and
Yong-Chun
Luo
and
Yong-Chun
Luo
 *
*
State Key Laboratory of Applied Organic Chemistry, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000, P. R. China. E-mail: luoych@lzu.edu.cn
First published on 14th July 2017
Abstract
A Lewis acid catalyzed [3+4]-annulation reaction between cyclopropane 1,1-diesters and anthranils has been developed. This annulation consists of a reaction sequence involving ring-opening/aromatization/nucleophilic addition. Thereinto, aromatization is the driving force for this annulation. Using this reaction, a series of 8-oxa-1-azabicyclo[3.2.1]octanes can be prepared conveniently with excellent diastereoselectivity.
In the past few years, donor–acceptor (D–A) cyclopropanes1 have attracted great attention, and a large number of [3+2]- and [3+3]-annulation reactions2,3 have been developed for the synthesis of five- and six-membered cyclic compounds. However, only a few intermolecular [3+4]-annulation reactions of D–A cyclopropanes were reported.4 In 2008, Ivanova and co-workers first reported the concerted [3+4]-cycloaddition of D–A cyclopropane with 1,3-diphenylisobenzofuran.4a In their work, 1,3-diphenylisobenzofuran acted as a diene to react with D–A cyclopropanes, the exo- and endo-products were obtained with a ratio from 53/47 to 86/14. Since then, the [3+4]-annulation reactions of D–A cyclopropanes with anthracenes,4b dienolsilyl ethers4c and ortho-bisthioquinone4d have been reported. These reactions showed that the [3+4]-annulation of D–A cyclopropane is an efficient strategy for the construction of seven-membered cyclic compounds.
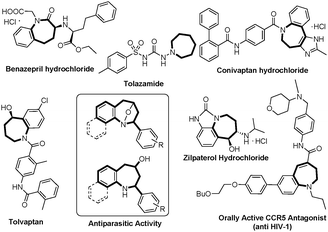
The seven-membered N-heterocyclic compounds, such as azepane and tetrahydro-1-benzazepine, are very important in medical chemistry and widely present in a variety of drugs and other bioactive molecules (Fig. 1).5 In view of our interest in the annulation reactions of D–A cyclopropanes,6 we thought that developing the [3+4]-annulation of D–A cyclopropane with azadiene or its equivalents would offer a direct method for the synthesis of heterocyclic compounds. Herein, we report the synthesis of 8-oxa-1-azabicyclo[3.2.1]octanes and tetrahydro-1-benzazepine derivatives via a diastereoselective [3+4]-annulation of D–A cyclopropanes with anthranils.7
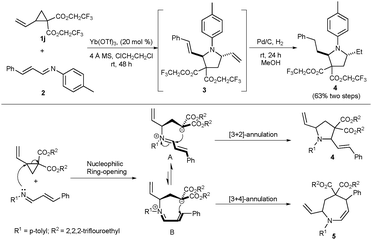
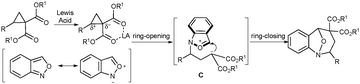
Initially, the annulation reaction between D–A cyclopropane 1j and azadiene 2 was examined under the catalysis of Yb(OTf)3 (Scheme 1). The reaction occurred smoothly at room temperature in 1,2-dichloroethane, but the purification of the annulation product was difficult. Therefore, the hydrogenation of the reaction mixture was carried out directly, and the polysubstituted pyrrolidine 4 was obtained as the major product. This result demonstrated that the [3+2]-annulation is the major pathway for the reaction. We thought that this may be ascribed to the difficult formation of zwitterion B from the linear azadiene 2 (Scheme 1). Then we turned our attention to the anthranil, a cyclic equivalent of azadiene, to react with D–A cyclopropanes (Scheme 2). We expected the opening of the activated cyclopropane by the nitrogen atom of anthranil, where the positive charge transfer could be driven by the aromatization to form the zwitterion C. Then, the annulation would occur via an intramolecular nucleophilic addition.
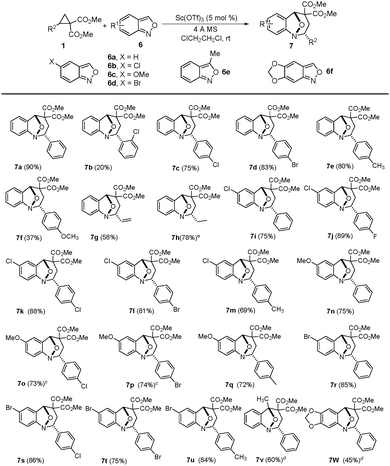
In order to test our hypothesis, the reaction between cyclopropane diester 1a and anthranil 6a was investigated, and the results are summarized in Table 1. To our satisfaction, when Yb(OTf)3 was used as a catalyst, the annulation reaction occurred in 1,2-dichloroethane at room temperature, and the expected product 7a was obtained in 83% yield as a single diastereoisomer (entry 1). To increase the yield, we evaluated several Lewis acids and discovered that Sc(OTf)3 was the most efficient catalyst for this reaction (entries 2–4). Under the catalysis of 10 mol% of Sc(OTf)3, the reaction completed in 1.5 hours, and the product 7a was obtained in 88% yield. Moreover, Ni(ClO4)2 and Hf(OTf)4 could also catalyze this reaction, but the reaction was slower. The solvent screening disclosed that 1,2-dichloroethane is the best solvent (entries 5 and 6). When reducing the catalyst loading to 5 mol%, the yield was increased slightly (entry 7). Finally, reducing the molar ratio of anthranil 6a to cyclopropane 1a and increasing the reaction concentration led to the optimal reaction conditions (entry 9), under which the product 7a was furnished in 90% yield in 1,2-dichloroethane in the presence of 5 mol% of Sc(OTf)3. The structure of 7a was determined by X-ray crystallographic analysis.
| Entry | Lewis acid | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.4 mmol), 2a (1.5 equiv., 0.6 mmol), Lewis acid (10 mol%, 0.04 mmol), solvent (C = 0.1 M, 4 mL), 4 Å molecular sieve (100 mg). b Isolated yield. c Sc(OTf)3 (5 mol%, 0.02 mmol). d 2a (1.2 equiv., 0.48 mmol). e Solvent (C = 0.2 M, 2 mL). | ||||
| 1 | Yb(OTf)3·xH2O | ClCH2CH2Cl | 12 | 83 |
| 2 | Sc(OTf)3 | ClCH2CH2Cl | 1.5 | 88 |
| 3 | Ni(ClO4)2·6H2O | ClCH2CH2Cl | 24 | 57 |
| 4 | Hf(OTf)4 | ClCH2CH2Cl | 24 | 41 |
| 5 | Sc(OTf)3 | CH2Cl2 | 1.5 | 84 |
| 6 | Sc(OTf)3 | Toluene | 8 | 86 |
| 7 | Sc(OTf)3c | ClCH2CH2Cl | 3 | 90 |
| 8d | Sc(OTf)3c | ClCH2CH2Cl | 6 | 85 |
| 9d,e | Sc(OTf)3c | ClCH2CH2Cl | 3 | 90 |
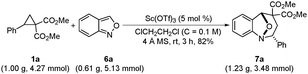
To investigate the scope of the reaction, a series of D–A cyclopropanes and anthranils were examined under the optimized conditions, and the results are summarized in Table 2. The 2-phenyl D–A cyclopropanes with an alkyl or a halogen on the phenyl gave the corresponding products in moderate to good yields (7i–7l and 7r–7u). However, when dimethyl 2-(4-methoxyphenyl)-cyclopropane 1,1-diester was used, the yield decreased dramatically (7f). In addition, the position of the substituent on the aryl group influenced the reactivity and the sterically demanding dimethyl 2-(2-chlorophenyl)-cyclopropane 1,1-diester gave the product (7b) in a lower yield. For aliphatic 2-vinyl and 2-ethyl D–A cyclopropanes, the corresponding products were also obtained in moderate yields (7g and 7h). On the other hand, the electron-rich anthranils showed lower reactivity. For example, 5-methoxyanthranil (6c) was less reactive than the 5-chloro or 5-bromoanthranils (6b and 6d) to give the corresponding products (7n, 7o and 7p) in lower yield. For the more electron-rich anthranil, 5,6-(methylenedioxy)anthranil (6f) was inactive that increasing the temperature and prolonging the reaction time offered the product in lower yield (7v). Apart from the electronic effect, the steric effect was another factor that influenced the reactivity. When 3-methylanthranil (6e) was used, the corresponding product (7v) was obtained only in 60% yield after 72 hours. Finally, a gram-scale reaction was carried out and the product 7a was obtained in 82% yield (Scheme 3). When increasing the catalyst loading to 10 mol%, the yield was improved to 86%.
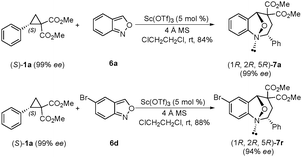
We next explored the stereospecificity of this [3+4]-annulation reaction with enantioenriched (99% ee) phenyl-substituted cyclopropane (S)-1a8 (Scheme 4). Anthranils 6a and 6d respectively reacted with (S)-1a with almost complete stereospecificity, giving (1R, 2R, 5R)-7a and (1R, 2R, 5R)-7r with 99% and 94% ee.9 It should also be noted that the configuration inversions of the stereocenter in (S)-1a were observed in this transformation.
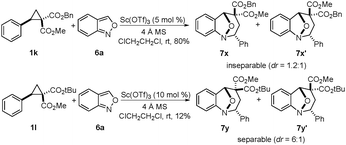
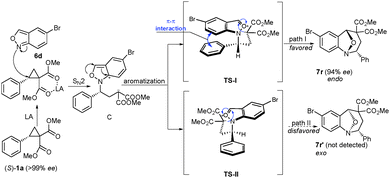
In order to understand the stereochemical outcome of the reaction, it was necessary to distinguish between the concerted and stepwise reaction mechanisms. For this purpose, the cyclopropanes 1k and 1l10,11 with different ester groups were used to react with anthranil 6a, respectively (Scheme 5). Cyclopropane 1k offered an inseparable mixture of 7x and 7x′ in 80% yield. When 1l was used, 7y and 7y′ were obtained in lower yield because of the steric hindrance. The structure of 7y was determined by X-ray crystallographic analysis. These results strongly suggest the possibility of the stepwise reaction mechanism.11 Based on above results, a plausible stepwise mechanism for this reaction is proposed (Scheme 6). First, an SN2-like nucleophilic attack of anthranil 6d to the activated D–A cyclopropane offers the zwitterion C, and the aromatization of zwitterion C occurs immediately. Then, the annulation is accomplished by an intramolecular nucleophilic addition of a carbanion to the oxonium ion via the transition state I or II. It is likely that the π–π interaction between the two aromatic rings in the transition state I leads to the endo-product with excellent diastereoselectivity.
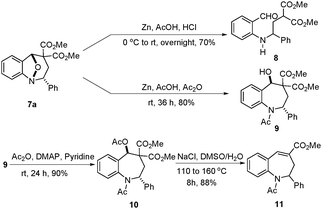
Finally, the derivatization of 8-oxa-1-azabicyclo[3.2.1]octanes was investigated (Scheme 7). First, we attempted to cleave the N–O bond in 7a using an activated zinc powder in a mixture of acetic acid and concentrated hydrochloric acid.12 Unfortunately, a retro-aldol reaction occurred after the N–O bond cleavage. To avoid this retro-aldol reaction, hydrochloric acid was replaced with acetic anhydride,13 then the reaction occurred smoothly and gave 2,3,4,5-tetrahydro-1-benzazepine 9 in high yield. After a sequence of acetylation and elimination, 2,3,4,5-tetrahydro-1-benzazepine 9 can be further transformed into 2,3-dihydro-1-benzazepine 11 in high yield.
In summary, we have developed a Lewis acid catalyzed [3+4]-annulation reaction of D–A cyclopropanes with anthranils. In this reaction, anthranils serve as azadienes to react with D–A cyclopropane via a nucleophilic ring-opening/aromatization/nucleophilic addition reaction sequence. Aromatization is the driving force to achieve this [3+4]-annulation. Using this reaction, a series of heterocyclic compounds bearing 1-benzazepine skeletons were readily prepared.
The authors declare no competing financial interest. We are grateful for the NSFC (21202070, 21402073), and the Fundamental Research Funds for the Central Universities (lzujbky-2016-54).
Notes and references
-
(a) H. U. Reissig, Top. Curr. Chem., 1988, 144, 73–135 CrossRef CAS
; (b) H. U. Reissig and R. Zimmer, Chem. Rev., 2003, 103, 1151–1196 CrossRef CAS PubMed
; (c) M. Yu and B. L. Pagenkopf, Tetrahedron, 2005, 61, 321–347 CrossRef CAS
; (d) T. F. Schneider, J. Kaschel and D. B. Werz, Angew. Chem., Int. Ed., 2014, 53, 5504–5523 CrossRef CAS PubMed
; (e) M. A. Cavitt, L. H. Phun and S. France, Chem. Soc. Rev., 2014, 43, 804–818 RSC
; (f) H. K. Grover, M. R. Emmett and M. A. Kerr, Org. Biomol. Chem., 2015, 13, 655–671 RSC
.
- Selected recent examples for [3+2] annulation:
(a) A. R. Rivero, I. Fernández, C. Ramírez de Arellano and M. A. Sierra, J. Org. Chem., 2015, 80, 1207–1213 CrossRef CAS PubMed
; (b) J. Liu, L. Zhou, W. Ye and C. Wang, Chem. Commun., 2014, 50, 9068–9071 RSC
; (c) F. de Nanteuil, E. Serrano, D. Perrotta and J. Waser, J. Am. Chem. Soc., 2014, 136, 6239–6242 CrossRef CAS PubMed
; (d) S. Chakrabarty, I. Chatterjee, B. Wibbeling, C. G. Daniliuc and A. Studer, Angew. Chem., Int. Ed., 2014, 53, 5964–5968 CrossRef CAS PubMed
; (e) W. Zhu, J. Fang, Y. Liu, J. Ren and Z. Wang, Angew. Chem., Int. Ed., 2013, 52, 2032–2037 CrossRef CAS PubMed
; (f) J.-P. Qu, Y. Liang, H. Xu, X.-L. Sun, Z.-X. Yu and Y. Tang, Chem. – Eur. J., 2012, 18, 2196–2201 CrossRef CAS PubMed
; (g) A. F. G. Goldberg, N. R. O'Connor, R. A. Craigll and B. M. Stoltz, Org. Lett., 2012, 14, 5314–5317 CrossRef CAS PubMed
; (h) A. T. Parsons, A. G. Smith, A. J. Neel and J. S. Johnson, J. Am. Chem. Soc., 2010, 132, 9688–9692 CrossRef CAS PubMed
; (i) A. T. Parsons and J. S. Johnson, J. Am. Chem. Soc., 2009, 131, 3122–3123 CrossRef CAS PubMed
.
- Selected recent examples of [3+3] annulation:
(a) S. Das, S. Chakrabarty, C. G. Daniliuc and A. Studer, Org. Lett., 2016, 18, 2784–2787 CrossRef CAS PubMed
; (b) L. K. B. Garve, M. Petzold, P. G. Jones and D. B. Werz, Org. Lett., 2016, 18, 564–567 CrossRef CAS PubMed
; (c) C. M. Braun, E. A. Congdon and K. A. Nolin, J. Org. Chem., 2015, 80, 1979–1984 CrossRef CAS PubMed
; (d) Q.-Q. Cheng, Y. Qian, P. Y. Zavalij and M. P. Doyle, Org. Lett., 2015, 17, 3568–3571 CrossRef CAS PubMed
.
-
(a) O. A. Ivanova, E. M. Budynina, Y. K. Grishin, I. V. Trushkov and P. V. Verteletskii, Angew. Chem., Int. Ed., 2008, 47, 1107–1110 CrossRef CAS PubMed
; (b) O. A. Ivanova, E. M. Budynina, Y. K. Grishin, I. V. Trushkov and P. V. Verteletskii, Eur. J. Org. Chem., 2008, 5329–5335 CrossRef CAS
; (c) H. Xu, J.-L. Hu, L. Wang, S. Liao and Y. Tang, J. Am. Chem. Soc., 2015, 137, 8006–8009 CrossRef CAS PubMed
; (d) L. K. B. Garve, M. Pawliczek, J. Wallbaum, P. G. Jones and D. B. Werz, Chem. – Eur. J., 2016, 22, 521–525 CrossRef CAS PubMed
.
- Selected recent examples:
(a) Y. Aramaki, M. Seto, T. Okawa, T. Oda, N. Kanzaki and M. Shiraishi, Chem. Pharm. Bull., 2004, 52, 254–258 CrossRef CAS PubMed
; (b) C. B. Breitenlechner, T. Wegge, L. Berillon, K. Graul, K. Marzenell, W.-G. Friebe, U. Thomas, R. Schumacher, R. Huber, R. A. Engh and B. Masjost, J. Med. Chem., 2004, 47, 1375–1390 CrossRef CAS PubMed
; (c) M. Seto, N. Miyamoto, K. Aikawa, Y. Aramaki, N. Kanzaki, Y. Iizawa, M. Baba and M. Shiraishi, Bioorg. Med. Chem., 2005, 13, 363–386 CrossRef CAS PubMed
; (d) M. Seto, K. Aikawa, N. Miyamoto, Y. Aramaki, N. Kanzaki, K. Takashima, Y. Kuze, Y. Iizawa, M. Baba and M. Shiraishi, J. Med. Chem., 2006, 49, 2037–2048 CrossRef CAS PubMed
; (e) S. Gómez-Ayala, J. A. Castrillón, A. Palma, S. M. Leal, P. Escobar and A. Bahsas, Bioorg. Med. Chem., 2010, 18, 4721–4739 CrossRef PubMed
.
-
(a) H.-H. Zhang, Y.-C. Luo, H.-P. Wang, W. Chen and P.-F. Xu, Org. Lett., 2014, 16, 4896–4899 CrossRef CAS PubMed
; (b) J.-Q. Han, H.-H. Zhang, P.-F. Xu and Y.-C. Luo, Org. Lett., 2016, 18, 5212–5215 CrossRef CAS PubMed
; (c) Y.-C. Luo, H. Ma, X.-Q. Hu and P.-F. Xu, J. Org. Chem., 2017, 82, 1013–1023 CrossRef CAS PubMed
.
- Selected recent examples for the application of anthranils in organic synthesis:
(a) S. Han, F. Vogt, J. A. May, S. Krishnan, M. Gatti, S. C. Virgil and B. M. Stoltz, J. Org. Chem., 2015, 80, 528–547 CrossRef CAS PubMed
; (b) S. Yu, Y. Li, X. Zhou, H. Wang, L. Kong and X. Li, Org. Lett., 2016, 18, 2812–2815 CrossRef CAS PubMed
; (c) L. Shi and B. Wang, Org. Lett., 2016, 18, 2820–2823 CrossRef CAS PubMed
; (d) M. Zou, J. Liu, C. Tang and N. Jiao, Org. Lett., 2016, 18, 3030–3033 CrossRef CAS PubMed
; (e) H. Jin, L. Huang, J. Xie, M. Rudolph, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2016, 55, 794–797 CrossRef CAS PubMed
; (f) C. Tang, M. Zou, J. Liu, X. Wen, X. Sun, Y. Zhang and N. Jiao, Chem. – Eur. J., 2016, 22, 11165–11169 CrossRef CAS PubMed
; (g) S. Yu, G. Tang, Y. Li, X. Zhou, Y. Lan and X. Li, Angew. Chem., Int. Ed., 2016, 55, 8696–8700 CrossRef CAS PubMed
; (h) W.-B. Shen, X.-Y. Xiao, Q. Sun, B. Zhou, X.-Q. Zhu, J.-Z. Yan, X. Lu and L.-W. Ye, Angew. Chem., Int. Ed., 2017, 56, 605–609 CrossRef CAS PubMed
.
- P. D. Pohlhaus, S. D. Sanders, A. T. Parsons, W. Li and J. S. Johnson, J. Am. Chem. Soc., 2008, 130, 8642–8650 CrossRef CAS PubMed
.
- Absolute configuration of (1R, 2R, 5R)-7r was determined by X-ray crystallographic analysis (please see in the ESI†).
- M. R. Emmett and M. A. Kerr, Org. Lett., 2011, 13, 4180–4183 CrossRef CAS PubMed
.
-
(a) A. O. Chagarovskiy, O. A. Ivanova, E. M. Budynina, E. L. Kolychev, M. S. Nechaev, I. V. Trushkov and M. Y. Melńikova, Russ. Chem. Bull., 2013, 62, 2407–2423 CrossRef CAS
; (b) L. Wang, Y. Murai, T. Yoshida, A. Ishida, K. Masuda, Y. Sakihama, Y. Hashidoko, Y. Hatanaka and M. Hashimoto, Org. Lett., 2015, 17, 616–619 CrossRef PubMed
.
- L. M. Acosta, A. Palma and A. Bahsas, Tetrahedron, 2010, 66, 8392–8401 CrossRef CAS
.
- S. W. Baldwin and A. Long, Org. Lett., 2004, 6, 1653–1656 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental details, characterization and NMR spectra of the products. CCDC 1540957 (7a), 1539625 ((1R, 2R, 5R)-7r), 1559038 (7y) and 1540954 (9). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7cc04239f |
| This journal is © The Royal Society of Chemistry 2017 |