Open Access Article
Open Access ArticlePd(0)-Catalyzed intramolecular arylative dearomatization of β-naphthols†
Ren-Qi
Xu
,
Ping
Yang
and
Shu-Li
You
 *
*
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032, China. E-mail: slyou@sioc.ac.cn
First published on 13th June 2017
Abstract
An efficient Pd(0)-catalyzed intramolecular arylative dearomatization of β-naphthols is described. Using Q-Phos as a ligand, the arylative dearomatization reaction proceeded smoothly affording excellent yields and chemoselectivity even when the catalyst loading was reduced to 0.1 mol%. This method offers an efficient access to a series of structurally diverse spirocarbocycles. Preliminary investigation indicates that an enantioselective reaction is feasible in the presence of a chiral phosphoramidite ligand.
Spirocarbocycles often exist in diverse natural products, biologically active molecules1 and useful chemical structures2 (Fig. 1). Therefore, it has attracted the intense attention of organic chemists to synthesize the unique structure of spirocarbocycles. Due to the congested quaternary carbon centers existed in spirocarbocycles, it has been a long-standing challenge to develop convenient synthetic methodologies. The recent progress in organometallic catalysis provides new solutions for the synthesis.3 However, highly efficient synthetic routes to obtain structurally diverse spirocarbocycles from readily available starting materials are still in great demand.
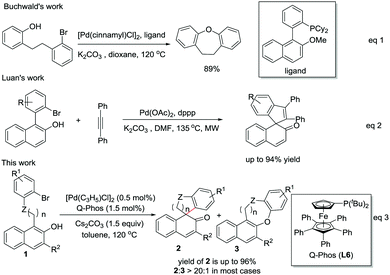
Phenol and its derivatives are important chemical starting materials and are widely used in organic synthesis.4 The recent development on the dearomatization of phenol and derivatives provides a novel route for the construction of spirocarbocycles.5,6 Of particular note, Pd-catalyzed cross-coupling type dearomatization reactions of anilines,7 phenols,8 indoles,9 pyrroles,10 pyridines11 and furans12 have been reported to construct interesting and useful structures. We envisaged that through an intramolecular design, spirocarbocycles can be easily obtained via the Pd-catalyzed arylative dearomatization of phenol derivatives. However, the chemoselectivity between the O-arylation and C-arylation reaction pathways is of great challenge in Pd-catalyzed dearomatization reactions especially when a quaternary carbon center is needed to be formed at the ortho-position of the phenolic hydroxyl group. For instance, the group of Buchwald reported that only the O-arylation product is obtained when an ortho-substituted phenol is subjected to Pd(0) catalysis (Scheme 1, eqn (1)).8b Recently, Luan and co-workers8e reported an elegant microwave-assisted Pd(0)-catalyzed alkyne migratory insertion and β-naphthol dearomatization cascade process, in which excellent chemoselectivity was achieved (Scheme 1, eqn (2)). However, to our knowledge, Pd(0)-catalyzed intramolecular direct arylative dearomatization of β-naphthols has not been reported. Herein, we describe an efficient Pd(0)-catalyzed intramolecular arylative dearomatization of β-naphthols affording all-carbon spirocarbocycle structures in excellent yields and chemoselectivity (Scheme 1, eqn (3)).
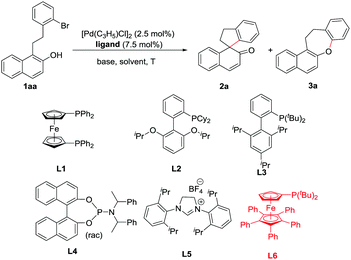
Initially, 1-(2-bromophenethyl)-2-naphthol (1aa) was chosen as a model substrate to examine different ligands under palladium catalysis. The results are summarized in Table 1. No desired dearomatized product was obtained when the diphosphine ligand dppf (L1) was used (Table 1, entry 1), while the utilization of PPh3 led to the formation of the desired dearomatized product (2a) in 24% NMR yield (Table 1, entry 2). With the Buchwald-type ligand RuPhos (L2), 2a was isolated in 11% yield together with the isolation of the O-arylation product in 9% yield (Table 1, entry 3). When the sterically bulky (di-tBu)XPhos (L3) was used as a ligand, only the O-arylation product was obtained (Table 1, entry 4). The utilization of the rac-Feringa ligand (L4) or SIPr·HBF4 (L5) led to the exclusive formation of 2a (56% yield, Table 1, entries 5 and 6). With Q-Phos (L6) as a ligand, 2a could be obtained in 68% yield with 3a isolated in 17% yield. Subsequently, several bases were examined (Table 1, entries 8–11). To our delight, the stronger inorganic base Cs2CO3 almost exclusively gave the desired dearomatized product (2a) in 91% yield within 1 hour. Lowering the catalyst loading to 0.5 mol% and 0.1 mol% led to slightly higher yields in both cases but a longer reaction time was needed (Table 1, entries 12 and 13). Notably, the reaction underwent smoothly even at 40 °C (Table 1, entry 14). The reaction in varied solvents, such as DCM, dioxane and THF, all gave satisfactory yields at 40 °C (92–95% yields, Table 1, entries 15–18). Considering the efficiency and convenience of the experiments, the optimized conditions were obtained as the following: 0.5 mol% [Pd(C3H5)Cl]2, 1.5 mol% Q-Phos (L6), and 1.5 equiv. of Cs2CO3 in toluene at 120 °C (Table 1, entry 12).
| Entry | Ligand | Base | Solvent | T (°C) | Time (h) | 2a yieldb (%) |
2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3ac 3ac |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: 1aa (0.2 mmol), [Pd(C3H5)Cl]2 (0.005 mmol), ligand (0.015 mmol), base (0.3 mmol) in solvent (1.0 mL), T °C. b Determined by 1H NMR using CH2Br2 (0.2 mmol) as an internal standard. c Determined by 1H NMR of the crude products. d Isolated yield. e [Pd(C3H5)Cl]2 (0.5 mol%) and L6 (1.5 mol%) were used. f [Pd(C3H5)Cl]2 (0.1 mol%) and L6 (0.3 mol%) were used. | |||||||
| 1 | L1 | K2CO3 | Toluene | 120 | 8 | 0 | ND |
| 2 | PPh3 | K2CO3 | Toluene | 120 | 8 | 24 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | L2 | K2CO3 | Toluene | 120 | 8 | 11d | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
| 4 | L3 | K2CO3 | Toluene | 120 | 8 | 0 | <20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | L4 | K2CO3 | Toluene | 120 | 8 | 56d | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | L5 | K2CO3 | Toluene | 120 | 8 | 56d | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | L6 | K2CO3 | Toluene | 120 | 8 | 68d | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | L6 | Na2CO3 | Toluene | 120 | 8 | Trace | ND |
| 9 | L6 | K3PO4 | Toluene | 120 | 8 | 64 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | L6 | DBU | Toluene | 120 | 10 | 13 | ND |
| 11 | L6 | Cs2CO3 | Toluene | 120 | 1 | 91d | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12e | L6 | Cs2CO3 | Toluene | 120 | 5 | 95d | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13f | L6 | Cs2CO3 | Toluene | 120 | 31 | 95d | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 14 | L6 | Cs2CO3 | Toluene | 40 | 7 | 92d | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 15 | L6 | Cs2CO3 | DCM | 40 | 7 | 93d | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 16 | L6 | Cs2CO3 | THF | 40 | 7 | 92 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 17 | L6 | Cs2CO3 | CH3CN | 40 | 7 | 20 | ND |
| 18 | L6 | Cs2CO3 | Dioxane | 40 | 7 | 95 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Next, we turned our attention to examine the substrate scope of this reaction. As shown in Scheme 2, substrates by cleaving different C–X bonds all gave their corresponding products in excellent yields and chemoselectivity (X = Cl, 92% yield, 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a = 14
3a = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; X = Br, 95% yield, 2a
1; X = Br, 95% yield, 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a > 20
3a > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; X = I, 94% yield, 2a
1; X = I, 94% yield, 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a = 20
3a = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Then, a wide range of substituted aryl bromides bearing either electron-donating or electron-withdrawing groups was tested. In all cases, the intramolecular dearomatization reaction proceeded smoothly to afford their corresponding products (2b–2g) in excellent yields (92–95%) and chemoselectivity. The structure of 2c was confirmed by X-ray crystallographic analysis. The reaction of the CF3-substituted substrate gave the desired product (2h) in good yield with slightly decreased chemoselectivity (86% yield, 2h
1). Then, a wide range of substituted aryl bromides bearing either electron-donating or electron-withdrawing groups was tested. In all cases, the intramolecular dearomatization reaction proceeded smoothly to afford their corresponding products (2b–2g) in excellent yields (92–95%) and chemoselectivity. The structure of 2c was confirmed by X-ray crystallographic analysis. The reaction of the CF3-substituted substrate gave the desired product (2h) in good yield with slightly decreased chemoselectivity (86% yield, 2h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3h = 12
3h = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Then the influence of the substituent group on the β-naphthol moiety was investigated. 3-Substituted (Me, CO2Me) naphthols were transformed into dearomatized products (2i and 2j) in good yields and chemoselectivity (80% yield, 2i
1). Then the influence of the substituent group on the β-naphthol moiety was investigated. 3-Substituted (Me, CO2Me) naphthols were transformed into dearomatized products (2i and 2j) in good yields and chemoselectivity (80% yield, 2i![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3i = 10
3i = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 82% yield, 2j
1; 82% yield, 2j![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3j = 8
3j = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Fortunately, the substituents on the other positions (6-Ph and 7-Ph) of the β-naphthol moiety did not influence the chemoselectivity to give 2k and 2l in excellent yields and chemoselectivity (95% yield, 2k
1). Fortunately, the substituents on the other positions (6-Ph and 7-Ph) of the β-naphthol moiety did not influence the chemoselectivity to give 2k and 2l in excellent yields and chemoselectivity (95% yield, 2k![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3k = 19
3k = 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 95% yield, 2l
1; 95% yield, 2l![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3l = 18
3l = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Apart from substrates bearing an all-carbon tether, the substrate with an N-linked tether (1m) was also compatible, affording the desired product (2m) in 74% yield and excellent chemoselectivity (2m
1). Apart from substrates bearing an all-carbon tether, the substrate with an N-linked tether (1m) was also compatible, affording the desired product (2m) in 74% yield and excellent chemoselectivity (2m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3m > 20
3m > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) under relatively milder conditions (at 60 °C). For substrate 1n with an extended carbon chain, the desired dearomatized product with a 6-membered ring formation (2n) was obtained smoothly in 96% yield and excellent chemoselectivity (2n
1) under relatively milder conditions (at 60 °C). For substrate 1n with an extended carbon chain, the desired dearomatized product with a 6-membered ring formation (2n) was obtained smoothly in 96% yield and excellent chemoselectivity (2n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3n > 20
3n > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Interestingly, the reaction of the α-naphthol substrate gave the dearomatized product (2o) in 88% yield with a 2o
1). Interestingly, the reaction of the α-naphthol substrate gave the dearomatized product (2o) in 88% yield with a 2o![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3o ratio of 20
3o ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
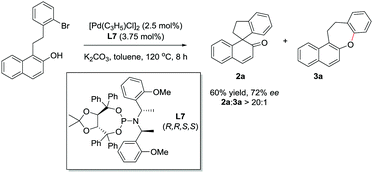
The asymmetric reaction was also explored. Several commercially available chiral phosphine ligands were screened. TADDOL-derived phosphoramidite (L7) was found to be the optimal ligand. In the presence of 2.5 mol% [Pd(C3H5)Cl]2 and 3.75 mol% L7, the reaction of 1aa could give 2a in 60% yield and 72% ee with excellent chemoselectivity (2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a > 20
3a > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Scheme 3, see the ESI† for details).
1, Scheme 3, see the ESI† for details).
To further demonstrate the utility of this method, a gram-scale reaction and several transformations of the 2-naphthalenone product have been carried out. The intramolecular dearomatization of 1aa on a 5.0 mmol scale gave the desired product 2a in 95% yield and excellent chemoselectivity (2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a = 21
3a = 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) while the catalyst loading could be further reduced to 0.1 mol% (Scheme 4). In addition, the ketone group of the product (2a) could be selectively reduced by LiAlH4 to afford the alcohol 4a in 93% yield (dr = 5
1) while the catalyst loading could be further reduced to 0.1 mol% (Scheme 4). In addition, the ketone group of the product (2a) could be selectively reduced by LiAlH4 to afford the alcohol 4a in 93% yield (dr = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), while the carbon–carbon double bond could be reduced by Pd/C catalyzed hydrogenation to afford 5a in 83% yield (Scheme 5).
1), while the carbon–carbon double bond could be reduced by Pd/C catalyzed hydrogenation to afford 5a in 83% yield (Scheme 5).
In summary, we have developed a Pd-catalyzed intramolecular dearomatization of β-naphthols to construct an all-carbon quaternary stereocenter at the ortho-position of the hydroxyl group with excellent yields and chemoselectivity. A series of structurally diverse spirocarbocycles were obtained efficiently and conveniently. Further studies on the application of the current method and development of more efficient catalytic asymmetric reactions are currently underway in our laboratory.
We thank the National Key Research and Development Program of China (2016YFA0202900), the National Basic Research Program of China (2015CB856600), the NSFC (21332009, 21361140373, and 21421091), the Program of Shanghai Subject Chief Scientist (16XD1404300), and the Chinese Academy of Sciences (XDB20000000, QYZDY-SSW-SLH012) for generous financial support.
Notes and references
- (a) Y.-L. Zhang, J. Zhang, N. Jiang, Y.-H. Lu, L. Wang, S.-H. Xu, W. Wang, G.-F. Zhang, Q. Xu, H. M. Ge, J. Ma, Y.-C. Song and R.-X. Tan, J. Am. Chem. Soc., 2011, 133, 5931 CrossRef CAS PubMed; (b) Z. Xi, G. B. Jones, G. Qabaja, J. Wright, F. Johnson and I. H. Goldberg, Org. Lett., 1999, 1, 1375 CrossRef CAS PubMed.
- (a) A. S. C. Chan, W.-H. Hu, C.-C. Pai, C.-P. Lau, Y.-Z. Jiang, A.-Q. Mi, M. Yan, J. Sun, R.-L. Lou and J.-G. Deng, J. Am. Chem. Soc., 1997, 119, 9570 CrossRef CAS; (b) J.-H. Zhang, J. Liao, X. Cui, K.-B. Yu, J.-G. Deng, S.-F. Zhu, L.-X. Wang, Q.-L. Zhou, L.-W. Chung and T. Ye, Tetrahedron: Asymmetry, 2002, 13, 1363 CrossRef CAS; (c) J.-H. Xie and Q.-L. Zhou, Acc. Chem. Res., 2008, 41, 581 CrossRef CAS PubMed.
- (a) A. Fürstner, Chem. Soc. Rev., 2009, 38, 3208 RSC; (b) V. A. D'yakonov, O. A. Trapeznikova, A. de Meijere and U. M. Dzhemilev, Chem. Rev., 2014, 114, 5775 CrossRef PubMed; (c) L. Fensterbank and M. Malacria, Acc. Chem. Res., 2014, 47, 953 CrossRef CAS PubMed.
- M. Weber, M. Weber and M. Kleine-Boymann, “Phenol” in Ullmanns Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2004 Search PubMed.
- For recent reviews, see: (a) A. R. Pape, K. P. Kaliappan and E. P. Kündig, Chem. Rev., 2000, 100, 2917 CrossRef CAS PubMed; (b) S. Quideau, L. Pouységu and D. Deffieux, Synlett, 2008, 467 CrossRef CAS; (c) L. Pouységu, D. Deffieux and S. Quideau, Tetrahedron, 2010, 66, 2235 CrossRef; (d) S. P. Roche and J. A. Porco Jr., Angew. Chem., Int. Ed., 2011, 50, 4068 CrossRef CAS PubMed; (e) C.-X. Zhuo, W. Zhang and S.-L. You, Angew. Chem., Int. Ed., 2012, 51, 12662 CrossRef PubMed; (f) Q. Ding, Y. Ye and R. Fan, Synthesis, 2013, 1 Search PubMed; (g) C.-X. Zhuo, C. Zheng and S.-L. You, Acc. Chem. Res., 2014, 47, 2558 CrossRef CAS PubMed; (h) W.-T. Wu, L. Zhang and S.-L. You, Chem. Soc. Rev., 2016, 45, 1570 RSC; (i) W. Sun, G. Li, L. Hong and R. Wang, Org. Biomol. Chem., 2016, 14, 2164 RSC; (j) C. Zheng and S.-L. You, Chem, 2016, 1, 830 CrossRef CAS; (k) R. B. Bedford, in Asymmetric Dearomatization Reactions, ed. S.-L. You, Wiley-VCH, Verlag GmbH & Co. KGaA, 2016, ch. 10, pp. 229–246 Search PubMed; (l) W.-T. Wu, L. Zhang and S.-L. You, Acta Chim. Sin., 2017, 75, 419 CrossRef CAS.
- For selected recent examples: (a) T. Dohi, A. Maruyama, N. Takenaga, K. Senami, Y. Minamitsuji, H. Fujioka, S. B. Caemmerer and Y. Kita, Angew. Chem., Int. Ed., 2008, 47, 3787 CrossRef CAS PubMed; (b) J. K. Boppisetti and V. B. Birman, Org. Lett., 2009, 11, 1221 CrossRef CAS PubMed; (c) S. Quideau, G. Lyvinec, M. Marguerit, K. Bathany, A. Ozanne-Beaudenon, T. Buffeteau, D. Cavagnat and A. Chénedé, Angew. Chem., Int. Ed., 2009, 48, 4605 CrossRef CAS PubMed; (d) M. Uyanik, T. Yasui and K. Ishihara, Angew. Chem., Int. Ed., 2010, 49, 2175 CrossRef CAS PubMed; (e) T. Yakura, M. Omoto, Y. Yamauchi, Y. Tian and A. Ozono, Tetrahedron, 2010, 66, 5833 CrossRef CAS; (f) T. Nemoto, Y. Ishige, M. Yoshida, Y. Kohno, M. Kanematsu and Y. Hamada, Org. Lett., 2010, 12, 5020 CrossRef CAS PubMed; (g) A. Rudolph, P. H. Bos, A. Meetsma, A. J. Minnaard and B. L. Feringa, Angew. Chem., Int. Ed., 2011, 50, 5834 CrossRef CAS PubMed; (h) T. Oguma and T. Katsuki, J. Am. Chem. Soc., 2012, 134, 20017 CrossRef CAS PubMed; (i) T. Nemoto, Z. Zhao, T. Yokosaka, Y. Suzuki, R. Wu and Y. Hamada, Angew. Chem., Int. Ed., 2013, 52, 2217 CrossRef CAS PubMed; (j) C.-X. Zhuo and S.-L. You, Angew. Chem., Int. Ed., 2013, 52, 10056 CrossRef CAS PubMed; (k) R. J. Phipps and F. D. Toste, J. Am. Chem. Soc., 2013, 135, 1268 CrossRef CAS PubMed; (l) J. Nan, Z. Zuo, L. Luo, L. Bai, H. Zheng, Y. Yuan, J. Liu, X. Luan and Y. Wang, J. Am. Chem. Soc., 2013, 135, 17306 CrossRef CAS PubMed; (m) C. Bosset, R. Coffinier, P. A. Peixoto, M. El Assal, K. Miqueu, J.-M. Sotiropoulos, L. Pouységu and S. Quideau, Angew. Chem., Int. Ed., 2014, 53, 9860 CrossRef CAS PubMed; (n) T. Nemoto, N. Matsuo and Y. Hamada, Adv. Synth. Catal., 2014, 356, 2417 CrossRef CAS; (o) S.-G. Wang, Q. Yin, C.-X. Zhuo and S.-L. You, Angew. Chem., Int. Ed., 2015, 54, 647 CAS; (p) D. Yang, L. Wang, F. Han, D. Li, D. Zhao and R. Wang, Angew. Chem., Int. Ed., 2015, 54, 2185 CrossRef CAS PubMed; (q) J. Zheng, S.-B. Wang, C. Zheng and S.-L. You, J. Am. Chem. Soc., 2015, 137, 4880 CrossRef CAS PubMed; (r) X. Lian, L. Lin, G. Wang, X. Liu and X. Feng, Chem. – Eur. J., 2015, 21, 17453 CrossRef CAS PubMed; (s) Q. Yin, S.-G. Wang, X.-W. Liang, D.-W. Gao, J. Zheng and S.-L. You, Chem. Sci., 2015, 6, 4179 RSC; (t) S.-G. Wang, X.-J. Liu, Q.-C. Zhao, C. Zheng, S.-B. Wang and S.-L. You, Angew. Chem., Int. Ed., 2015, 54, 14929 CrossRef CAS PubMed; (u) W.-T. Wu, R.-Q. Xu, L. Zhang and S.-L. You, Chem. Sci., 2016, 7, 3427 RSC; (v) Q. Cheng, Y. Wang and S.-L. You, Angew. Chem., Int. Ed., 2016, 55, 3496 CrossRef CAS PubMed; (w) H.-F. Tu, C. Zheng, R.-Q. Xu, X.-J. Liu and S.-L. You, Angew. Chem., Int. Ed., 2017, 56, 3237 CrossRef CAS PubMed; (x) D. Shen, Q. Chen, P. Yan, X. Zeng and G. Zhong, Angew. Chem., Int. Ed., 2017, 56, 3242 CrossRef CAS PubMed.
- (a) J. Garcia-Fortanet, F. Kessler and S. L. Buchwald, J. Am. Chem. Soc., 2009, 131, 6676 CrossRef CAS PubMed; (b) R. B. Bedford, C. P. Butts, M. F. Haddow, R. Osborne and R. F. Sankey, Chem. Commun., 2009, 4832 RSC.
- (a) S. Wiegand and H. J. Schäfer, Tetrahedron, 1995, 51, 5341 CrossRef CAS; (b) S. Rousseaux, J. Garcia-Fortanet, M. A. D. A. Sanchez and S. L. Buchwald, J. Am. Chem. Soc., 2011, 133, 9282 CrossRef CAS PubMed; (c) R.-Q. Xu, Q. Gu, W.-T. Wu, Z.-A. Zhao and S.-L. You, J. Am. Chem. Soc., 2014, 136, 15469 CrossRef CAS PubMed; (d) K. Du, P. Guo, Y. Chen, Z. Cao, Z. Wang and W. Tang, Angew. Chem., Int. Ed., 2015, 54, 3033 CrossRef CAS PubMed; (e) H. Zheng, L. Bai, J. Liu, J. Nan, Z. Zuo, L. Yang, Y. Wang and X. Luan, Chem. Commun., 2015, 51, 3061 RSC; (f) L. Yang, H. Zheng, L. Luo, J. Nan, J. Liu, Y. Wang and X. Luan, J. Am. Chem. Soc., 2015, 137, 4876 CrossRef CAS PubMed; (g) L. Bai, Y. Yuan, J. Liu, J. Wu, L. Han, H. Wang, Y. Wang and X. Luan, Angew. Chem., Int. Ed., 2016, 55, 6946 CrossRef CAS PubMed; (h) R.-Q. Xu, P. Yang, H.-F. Tu, S.-G. Wang and S.-L. You, Angew. Chem., Int. Ed., 2016, 55, 15137 CrossRef CAS PubMed; (i) Z. Zuo, H. Wang, L. Fan, J. Liu, Y. Wang and X. Luan, Angew. Chem., Int. Ed., 2017, 56, 2767 CrossRef CAS PubMed; (j) G. Zhao, G. Xu, C. Qian and W. Tang, J. Am. Chem. Soc., 2017, 139, 3360 CrossRef CAS PubMed; (k) R.-Q. Xu, Q. Gu and S.-L. You, Angew. Chem., Int. Ed., 2017, 56, 7252 CrossRef CAS PubMed.
- (a) R. B. Bedford, N. Fey, M. F. Haddow and R. F. Sankey, Chem. Commun., 2011, 47, 3649 RSC; (b) L. Zhao, Z. Li, L. Chang, J. Xu, H. Yao and X. Wu, Org. Lett., 2012, 14, 2066 CrossRef CAS PubMed; (c) K.-J. Wu, L.-X. Dai and S.-L. You, Org. Lett., 2012, 14, 3772 CrossRef CAS PubMed; (d) C. Shen, R.-R. Liu, R.-J. Fan, Y.-L. Li, T.-F. Xu, J.-R. Gao and Y.-X. Jia, J. Am. Chem. Soc., 2015, 137, 4936 CrossRef CAS PubMed; (e) D. A. Petrone, A. Yen, N. Zeidan and M. Lautens, Org. Lett., 2015, 17, 4838 CrossRef CAS PubMed; (f) D. A. Petrone, M. Kondo, N. Zeidan and M. Lautens, Chem. – Eur. J., 2016, 22, 5684 CrossRef CAS PubMed; (g) K. Douki, H. Ono, T. Taniguchi, J. Shimokawa, M. Kitamura and T. Fukuyama, J. Am. Chem. Soc., 2016, 138, 14578 CrossRef CAS PubMed; (h) R.-R. Liu, Y.-G. Wang, Y.-L. Li, B.-B. Huang, R.-X. Liang and Y.-X. Jia, Angew. Chem., Int. Ed., 2017, 56, 7475 CrossRef CAS PubMed.
- K.-J. Wu, L.-X. Dai and S.-L. You, Chem. Commun., 2013, 49, 8620 RSC.
- T. Xu and H. Alper, Org. Lett., 2015, 17, 1569 CrossRef CAS PubMed.
- (a) B. Yin, C. Cai, G. Zeng, R. Zhang, X. Li and H. Jiang, Org. Lett., 2012, 14, 1098 CrossRef CAS PubMed; (b) J. Li, H. Peng, F. Wang, X. Wang, H. Jiang and B. Yin, Org. Lett., 2016, 18, 3226 CrossRef CAS PubMed; (c) J. Liu, H. Peng, L. Lu, X. Xu, H. Jiang and B. Yin, Org. Lett., 2016, 18, 6440 CrossRef CAS PubMed; (d) J. Liu, X. Xu, J. Li, B. Liu, H. Jiang and B. Yin, Chem. Commun., 2016, 52, 9550 RSC; (e) J. Liu, H. Peng, Y. Yang, H. Jiang and B. Yin, J. Org. Chem., 2016, 81, 9695 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1534422. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7cc04022a |
| This journal is © The Royal Society of Chemistry 2017 |