Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Record-high thermal barrier of the relaxation of magnetization in the nitride clusterfullerene Dy2ScN@C80-Ih†
D. S.
Krylov‡
,
F.
Liu‡
 ,
S. M.
Avdoshenko
,
S. M.
Avdoshenko
 ,
L.
Spree
,
B.
Weise
,
A.
Waske
,
L.
Spree
,
B.
Weise
,
A.
Waske
 ,
A. U. B.
Wolter
,
B.
Büchner
and
A. A.
Popov
,
A. U. B.
Wolter
,
B.
Büchner
and
A. A.
Popov
 *
*
Leibniz Institute for Solid State and Materials Research, 01069 Dresden, Germany. E-mail: a.popov@ifw-dresden.de
First published on 16th June 2017
Abstract
The Dy-Sc nitride clusterfullerene Dy2ScN@C80-Ih exhibits slow relaxation of magnetization up to 76 K. Above 60 K, thermally-activated relaxation proceeds via the fifth-excited Kramers doublet with the energy of 1735 ± 21 K, which is the highest barrier ever reported for dinuclear lanthanide single molecule magnets.
The encapsulation of metal clusters within the fullerene cage has multiple structural and physical consequences for the properties of endohedral metallofullerenes (EMFs).1 In particular, unusual magnetic properties can be achieved in EMFs when encapsulated metals are lanthanides. The interaction of lanthanide ions with negatively-charged non-metal ions (nitride, carbide, oxide) within the endohedral cluster leads to a large magnetic anisotropy of the former,2 which is crucial for the single molecule magnetism (SMMs are the molecules with bistable magnetic ground state showing slow relaxation of the magnetization).3,4
DySc2N@C80-Ih was the first EMF to show SMM properties.5 In later reports, Dy2ScN@C80-Ih6 as well as isostructural Dy2TiC@C807 were found to exhibit SMM behaviour. A slow relaxation of magnetization was also reported in nitride and carbonitride clusterfullerenes with non-Kramers Ho and Tb ions,8 but with much shorter relaxation times than in Dy-EMFs.
The high magnetic anisotropy of Dy ions in EMFs is expected to lead to large barriers of the thermally-activated relaxation of magnetization via the Orbach relaxation mechanism. Theoretical predictions for such barriers exceed 1000 K and approach 2000 K.2a,d However, such barriers have not been observed experimentally yet, which may be caused by the limited temperature ranges, in which magnetic properties of EMFs were studied. For Dy2ScN@C80, which is the best EMF-SMM reported so far, detailed magnetic studies have been limited to temperatures below 20 K.6 They revealed an Orbach relaxation mechanism with the barrier of 8.5 K assigned to the exchange/dipolar excited state, in which Dy ions are coupled antiferromagnetically. In this work we use ac magnetometry to unravel the mechanism of the relaxation of magnetization in Dy2ScN@C80 up to 76 K and find that the compound has an unprecedentedly high relaxation barrier exceeding all reported values for polynuclear systems and approaching the highest value of 1815(1) K reported for the single-ion Dy-SMM.4c
Dy2ScN@C80-Ih has been synthesized by arc-discharge synthesis as described previously.9 In brief, a mixture of Dy2O3, Sc2O3, and guanidine thiocyanate was mixed with graphite powder and packed into the drilled-out graphite rods, which were then evaporated in 180 mbar He atmosphere. EMFs were Soxhlet-extracted from the resulting soot by CS2 and separated by HPLC (see ESI† for further details).
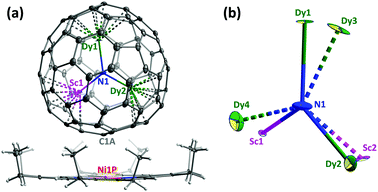
In the previous work, the molecular structure of Dy2ScN@C80 was established by spectroscopic techniques.9b Here we report on the crystallographic elucidation of its molecular structure. A cocrystal Dy2ScN@C80-Ih·NiII(OEP)·2C6H6 (OEP = octaethylporphyrin) was obtained by layering a benzene solution of NiII(OEP) over the solution of Dy2ScN@C80-Ih in carbon disulphide.§10Fig. 1a displays the relative orientation of the fullerene and NiII(OEP) molecules in the crystal. The nearest cage-Ni contact (between Ni1P and C1A) is determined to be 2.793(7) Å. Thanks to the coordination to the bowl-shaped NiII(OEP), the C80-Ih(7) cage is fully ordered, whereas the Dy2ScN cluster is disordered between two sites with fractional occupancies of 0.69 and 0.31. DFT calculations for Y2ScN@C80-Ih (see ESI†) show that both sites correspond to energy minima with the energy difference of 6.3 kJ mol−1 in favour of the more abundant configuration. In both sites, Sc and one of the Dy ions are directed towards NiII(OEP), whereas the second Dy is facing the opposite side of the cage. A similar arrangement of the cluster was reported in the Gd2ScN@C80-Ih NiII(OEP)·2C6H6 crystal.11 Furthermore, this arrangement follows the general pattern observed for M3N@C80-Ih12 and M2TiC@C80-Ih13 clusterfullerenes co-crystallized with NiII(OEP). Since only the Ih isomer is considered in this work, in the following we will omit the designation of the fullerene cage.
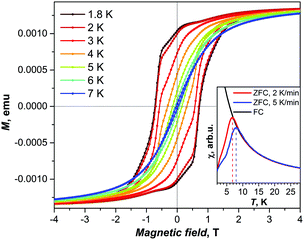
The magnetic properties of a Dy2ScN@C80 powder sample were studied by SQUID magnetometry. Fig. 2 shows the magnetization curves measured at low temperatures. When the magnetic field is swept with a rate of 2.9 mT s−1, the hysteresis of the magnetization is observed between 1.8 and 7 K (Fig. 2; the coercive field at 2 K is 0.7 T). The blocking temperature of magnetization, TB, is defined as the position of the peak on the χ–T curve of a zero-field-cooled sample (χ is the magnetic susceptibility). For Dy2ScN@C80, TB depends on the temperature sweep rate and varies between 7 and 8 K when the rate is increased from 2 K min−1 to 5 K min−1 (Fig. 2, inset).
The magnetization relaxation times below 5 K can be determined from the decay of the magnetization measured with dc-magnetometry. Unfortunately, determination of the relaxation time in such measurements is rather ambiguous as relaxation curves at low temperature usually show a form of multiexponential decay. In a previous work, a bi-exponential fitting was used and the longer times were interpreted as intrinsic to the SMM.6 Here we use a stretched exponential fitting to obtain average relaxation times. The obtained relaxation times follow an Arrhenius behaviour corresponding to the Orbach relaxation process:
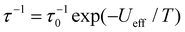 | (1) |
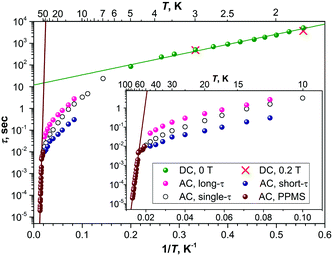
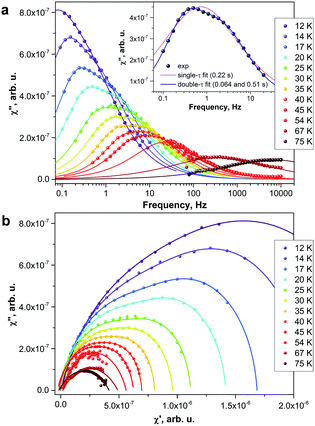
Above 5 K, the rates of the relaxation of magnetization were studied with ac magnetometry. In these measurements, we used two Quantum Design magnetometers, MPMS XL (with a reliable sensitivity from 0.1 Hz to ca. 500 Hz) and a PPMS system (frequency range 10 Hz–10 kHz, but with a rather poor sensitivity below 100 Hz). In the temperature range of 12–45 K, the measurements revealed distorted χ′′ peaks indicating that the relaxation of magnetization proceeds via two channels with distinct characteristic times (Fig. 4). The data were then fitted using either one or two relaxation times (discussed hereafter as single-τ and double-τ models, respectively; see inset in Fig. 4a). The single-τ model gives an average time of the two relaxation processes. Both short-τ and long-τ processes are temperature dependent. Interestingly, the long-τ relaxation channel dominates at lower temperatures, whereas an increase of the statistical weight of the short-τ relaxation channel is observed at higher temperatures (Fig. 3). Note that the local coordination sphere of the two Dy ions in the Dy2ScN@C80 molecule is slightly different, and hence the coexistence of the two concomitant relaxation processes may be caused by a different relaxation behaviour of Dy centres in one molecule. The coexistence of at least two relaxation channels is often observed in di- and polynuclear SMMs with non-equivalent lanthanide centres.3b,14 The nature of the relaxation mechanisms for these processes is not clear yet. Traditionally, sub-barrier relaxation in SMMs is ascribed to the Raman mechanism, but the recent analysis of spin-phonon coupling and dynamics by Lunghi et al. suggested that low-frequency unharmonic phonons with finite linewidth may cause Arrhenius-like behaviour at low temperatures.15
Above 45 K, the two relaxation processes cannot be distinguished anymore, and the single-τ behaviour is observed up to 76 K (above this temperature, the peak in χ′′ is beyond the accessible frequency range). Between 63 and 76 K, the data points show an Arrhenius behaviour (Fig. 3) with the effective energy barrier of 1735 ± 21 K and an attempt time τ0 = 2.39 × 10−15 s. Thus, Dy2ScN@C80 has one of the highest magnetization relaxation barriers ever reported for SMMs and is second to only [Dy(OtBu)2(py)5][BPh4] with the energy barrier of 1815(1) K.4c Before this work, the highest thermal relaxation barriers among dinuclear lanthanide systems were found in isocarbonyl-ligated Dy-metallocene [Cp*2Dy-{μ-(OC)2FeCp}]2 (953 K)16 and the hydroxide-bridged five-coordinate DyIII dimer (721 K).17
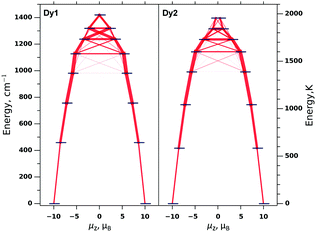
In weakly-coupled dinuclear SMMs, the relaxation of magnetization is believed to proceed via single-ion states.14d,18 To clarify the relaxation mechanism and the nature of the observed barrier in Dy2ScN@C80, we performed ab initio computations. As the apparent geometry of the Dy2ScN cluster in the crystal is distorted by the disorder, we used coordinates from the X-ray structure only as a starting point for the DFT geometry optimization of the Y2ScN@C80 molecule.¶ One of the Y ions in the optimized structure was then replaced by Dy, and single-point CASSCF/RASSI/ANO-RCC-VDZ calculations were performed using the Molcas 8.0 code.19 The crystal-field (CF) parameters derived by the SINGLE-ANISO module20 were then used in the PHI code21 for further model Hamiltonian calculations. Fig. 5 shows the CF energy levels of Dy1 and Dy2 as well as transition probabilities between the CF states. Similar to the earlier studies,2a our calculations predict a large CF splitting reaching 1400 cm−1 for both Dy ions. The CF is highly axial and gives 8 Kramers doublets (KDs) with the ground state corresponding to Jz = ±15/2. Several higher energy KDs also have almost pure mJ composition. As a result, transition probabilities within the KDs are very low up to the fourth CF excited state. At higher energies, mJ states mix significantly, leading to the dramatic increase of the transition probabilities (Fig. 5). Based on these calculations, the relaxation of the magnetization in Dy2ScN@C80 is expected to proceed efficiently via the fifth KDs of individual Dy centres. The computed energies of these states (1618 K for Dy1 and 1641 K for Dy2) are only slightly lower than the experimental value of 1735 K. The deviation is likely to be due to the insufficient accuracy of the CASSCF model (such as its lack of dynamic correlation), but may be also caused by other relaxation channels (e.g., the relaxation via the sixth or higher CF states).
To conclude, this Communication reports on the studies of the dynamic magnetic properties of Dy2ScN@C80 and reveals that it has a very high relaxation barrier of magnetization, 1735 ± 21 K. Based on ab initio calculations, the barrier is assigned to a thermally-activated relaxation via the fifth crystal-field excited state of individual Dy centres. High axiality of the CF states is crucial for reaching high barriers of the relaxation of magnetization as it prevents the relaxation via low-energy KDs.22 In Dy2ScN@C80, despite the low symmetry of the Dy coordination sphere, the high axiality is achieved because of the short distance between Dy and the nitride ion. Further increase of the barrier in Dy–nitride clusterfullerenes may be thus achieved by geometrically forcing the Dy–N distance to be shorter by either substituting Sc by a larger diamagnetic ion, or by considering a smaller carbon cage.
The authors acknowledge funding by the European Research Council under the European Union's Horizon 2020 research and innovation programme (grant no 648295 “GraM3”). Computational resources were provided by the Center for High Performance Computing at the TU Dresden. Manfred Weiss and Karine M. Röwer are acknowledged for the help with crystallographic measurements at BESSY.
Notes and references
- (a) A. A. Popov, S. Yang and L. Dunsch, Chem. Rev., 2013, 113, 5989–6113 CrossRef CAS PubMed; (b) X. Lu, L. Feng, T. Akasaka and S. Nagase, Chem. Soc. Rev., 2012, 41, 7723–7760 RSC; (c) A. Rodriguez-Fortea, A. L. Balch and J. M. Poblet, Chem. Soc. Rev., 2011, 40, 3551–3563 RSC.
- (a) V. Vieru, L. Ungur and L. F. Chibotaru, J. Phys. Chem. Lett., 2013, 4, 3565–3569 CrossRef CAS; (b) F. Cimpoesu, N. Dragoe, H. Ramanantoanina, W. Urland and C. Daul, Phys. Chem. Chem. Phys., 2014, 16, 11337–11348 RSC; (c) Y. Zhang, D. Krylov, M. Rosenkranz, S. Schiemenz and A. A. Popov, Chem. Sci., 2015, 6, 2328–2341 RSC; (d) M. K. Singh and G. Rajaraman, Chem. Commun., 2016, 52, 14047–14050 RSC.
- (a) R. Sessoli, D. Gatteschi, A. Caneschi and M. A. Novak, Nature, 1993, 365, 141–143 CrossRef CAS; (b) F. Habib and M. Murugesu, Chem. Soc. Rev., 2013, 42, 3278–3288 RSC; (c) P. Zhang, L. Zhang and J. Tang, Dalton Trans., 2015, 44, 3923–3929 RSC; (d) D. N. Woodruff, R. E. P. Winpenny and R. A. Layfield, Chem. Rev., 2013, 113, 5110–5148 CrossRef CAS PubMed; (e) J. Luzon and R. Sessoli, Dalton Trans., 2012, 41, 13556–13567 RSC; (f) L. Sorace, C. Benelli and D. Gatteschi, Chem. Soc. Rev., 2011, 40, 3092–3104 RSC; (g) S. T. Liddle and J. van Slageren, Chem. Soc. Rev., 2015, 44, 6655–6669 RSC; (h) J. D. Rinehart and J. R. Long, Chem. Sci., 2011, 2, 2078–2085 RSC.
- (a) S. K. Gupta, T. Rajeshkumar, G. Rajaraman and R. Murugavel, Chem. Sci., 2016, 7, 5181–5191 RSC; (b) Y.-C. Chen, J.-L. Liu, L. Ungur, J. Liu, Q.-W. Li, L.-F. Wang, Z.-P. Ni, L. F. Chibotaru, X.-M. Chen and M.-L. Tong, J. Am. Chem. Soc., 2016, 138, 2829–2837 CrossRef CAS PubMed; (c) Y.-S. Ding, N. F. Chilton, R. E. P. Winpenny and Y.-Z. Zheng, Angew. Chem., Int. Ed., 2016, 55, 16071–16074 CrossRef CAS PubMed.
- R. Westerström, J. Dreiser, C. Piamonteze, M. Muntwiler, S. Weyeneth, H. Brune, S. Rusponi, F. Nolting, A. Popov, S. Yang, L. Dunsch and T. Greber, J. Am. Chem. Soc., 2012, 134, 9840–9843 CrossRef PubMed.
- R. Westerström, J. Dreiser, C. Piamonteze, M. Muntwiler, S. Weyeneth, K. Krämer, S.-X. Liu, S. Decurtins, A. Popov, S. Yang, L. Dunsch and T. Greber, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 060406 CrossRef.
- K. Junghans, C. Schlesier, A. Kostanyan, N. A. Samoylova, Q. Deng, M. Rosenkranz, S. Schiemenz, R. Westerström, T. Greber, B. Büchner and A. A. Popov, Angew. Chem., Int. Ed., 2015, 54, 13411–13415 CrossRef CAS PubMed.
- (a) J. Dreiser, R. Westerström, Y. Zhang, A. A. Popov, L. Dunsch, K. Krämer, S.-X. Liu, S. Decurtins and T. Greber, Chem. – Eur. J., 2014, 20, 13536–13540 CrossRef CAS PubMed; (b) F. Liu, S. Wang, C.-L. Gao, Q. Deng, X. Zhu, A. Kostanyan, R. Westerström, F. Jin, S.-Y. Xie, A. A. Popov, T. Greber and S. Yang, Angew. Chem., Int. Ed., 2017, 56, 1830–1834 CrossRef CAS PubMed; (c) F. Liu, C.-L. Gao, Q. Deng, X. Zhu, A. Kostanyan, R. Westerström, S. Wang, Y.-Z. Tan, J. Tao, S.-Y. Xie, A. A. Popov, T. Greber and S. Yang, J. Am. Chem. Soc., 2016, 138, 14764–14771 CrossRef CAS PubMed.
- (a) T. Wei, F. Liu, S. Wang, X. Zhu, A. A. Popov and S. Yang, Chem. – Eur. J., 2015, 21, 5750–5759 CrossRef CAS PubMed; (b) S. Yang, A. A. Popov, C. Chen and L. Dunsch, J. Phys. Chem. C, 2009, 113, 7616–7623 CrossRef CAS.
- M. M. Olmstead, D. A. Costa, K. Maitra, B. C. Noll, S. L. Phillips, P. M. Van Calcar and A. L. Balch, J. Am. Chem. Soc., 1999, 121, 7090–7097 CrossRef CAS.
- S. Stevenson, C. Chancellor, H. M. Lee, M. M. Olmstead and A. L. Balch, Inorg. Chem., 2008, 47, 1420–1427 CrossRef CAS PubMed.
- (a) S. Stevenson, C. B. Rose, J. S. Maslenikova, J. R. Villarreal, M. A. Mackey, B. Q. Mercado, K. Chen, M. M. Olmstead and A. L. Balch, Inorg. Chem., 2012, 51, 13096–13102 CrossRef CAS PubMed; (b) X. L. Wang, T. M. Zuo, M. M. Olmstead, J. C. Duchamp, T. E. Glass, F. Cromer, A. L. Balch and H. C. Dorn, J. Am. Chem. Soc., 2006, 128, 8884–8889 CrossRef CAS PubMed; (c) T. Zuo, M. M. Olmstead, C. M. Beavers, A. L. Balch, G. Wang, G. T. Yee, C. Shu, L. Xu, B. Elliott, L. Echegoyen, J. C. Duchamp and H. C. Dorn, Inorg. Chem., 2008, 47, 5234–5244 CrossRef CAS PubMed.
- (a) K. Junghans, K. B. Ghiassi, N. A. Samoylova, Q. Deng, M. Rosenkranz, M. M. Olmstead, A. L. Balch and A. A. Popov, Chem. – Eur. J., 2016, 22, 13098–13107 CrossRef CAS PubMed; (b) A. L. Svitova, K. Ghiassi, C. Schlesier, K. Junghans, Y. Zhang, M. Olmstead, A. Balch, L. Dunsch and A. A. Popov, Nat. Commun., 2014, 5, 3568 CAS.
- (a) S.-Y. Lin, Y.-N. Guo, Y. Guo, L. Zhao, P. Zhang, H. Ke and J. Tang, Chem. Commun., 2012, 48, 6924–6926 RSC; (b) J.-D. Leng, J.-L. Liu, Y.-Z. Zheng, L. Ungur, L. F. Chibotaru, F.-S. Guo and M.-L. Tong, Chem. Commun., 2013, 49, 158–160 RSC; (c) P.-H. Lin, W.-B. Sun, M.-F. Yu, G.-M. Li, P.-F. Yan and M. Murugesu, Chem. Commun., 2011, 47, 10993–10995 RSC; (d) Y.-N. Guo, G.-F. Xu, W. Wernsdorfer, L. Ungur, Y. Guo, J. Tang, H.-J. Zhang, L. F. Chibotaru and A. K. Powell, J. Am. Chem. Soc., 2011, 133, 11948–11951 CrossRef CAS PubMed; (e) R. J. Blagg, L. Ungur, F. Tuna, J. Speak, P. Comar, D. Collison, W. Wernsdorfer, E. J. L. McInnes, L. F. Chibotaru and R. E. P. Winpenny, Nat. Chem., 2013, 5, 673–678 CrossRef CAS PubMed.
- A. Lunghi, F. Totti, R. Sessoli and S. Sanvito, Nat. Commun., 2017, 8, 14620 CrossRef PubMed.
- T. Pugh, N. F. Chilton and R. A. Layfield, Angew. Chem., Int. Ed., 2016, 55, 11082–11085 CrossRef CAS PubMed.
- J. Xiong, H.-Y. Ding, Y.-S. Meng, C. Gao, X.-J. Zhang, Z.-S. Meng, Y.-Q. Zhang, W. Shi, B.-W. Wang and S. Gao, Chem. Sci., 2017, 8, 1288–1294 RSC.
- Y. Wang, X.-L. Li, T.-W. Wang, Y. Song and X.-Z. You, Inorg. Chem., 2010, 49, 969–976 CrossRef CAS PubMed.
- F. Aquilante, J. Autschbach, R. K. Carlson, L. F. Chibotaru, M. G. Delcey, L. De Vico, I. Fdez. Galván, N. Ferré, L. M. Frutos, L. Gagliardi, M. Garavelli, A. Giussani, C. E. Hoyer, G. Li Manni, H. Lischka, D. Ma, P. Å. Malmqvist, T. Müller, A. Nenov, M. Olivucci, T. B. Pedersen, D. Peng, F. Plasser, B. Pritchard, M. Reiher, I. Rivalta, I. Schapiro, J. Segarra-Martí, M. Stenrup, D. G. Truhlar, L. Ungur, A. Valentini, S. Vancoillie, V. Veryazov, V. P. Vysotskiy, O. Weingart, F. Zapata and R. Lindh, J. Comput. Chem., 2016, 37, 506–541 CrossRef CAS PubMed.
- L. F. Chibotaru and L. Ungur, J. Chem. Phys., 2012, 137, 064112 CrossRef CAS PubMed.
- N. F. Chilton, R. P. Anderson, L. D. Turner, A. Soncini and K. S. Murray, J. Comput. Chem., 2013, 34, 1164–1175 CrossRef CAS PubMed.
- L. Ungur and L. F. Chibotaru, Inorg. Chem., 2016, 55, 10043–10056 CrossRef CAS PubMed.
- U. Mueller, R. Förster, M. Hellmig, F. U. Huschmann, A. Kastner, P. Malecki, S. Pühringer, M. Röwer, K. Sparta, M. Steffien, M. Ühlein, P. Wilk and M. S. Weiss, Eur. Phys. J. Plus, 2015, 130, 141 CrossRef.
- K. M. Sparta, M. Krug, U. Heinemann, U. Mueller and M. S. Weiss, J. Appl. Crystallogr., 2016, 49, 1085–1092 CAS.
- G. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 CrossRef PubMed.
- D. N. Laikov and Y. A. Ustynuk, Russ. Chem. Bull., 2005, 54, 820–826 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional experimental details, HPLC separation, magnetic measurement. CCDC 1547067. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7cc03580b |
| ‡ These authors contributed equally. |
| § After the two solutions diffused together over a period of one month, small black crystals (0.2 × 0.1 × 0.1 mm3) formed. X-ray diffraction data collection was carried out at 100 K at the BESSY storage ring (BL14.3, Berlin-Adlershof, Germany)23 using a MAR225 CCD detector, λ = 0.89429 Å. Diffraction data was processed with XDSAPP2.0 suite.24 The structure was solved by direct methods and refined using all data (based on F2) by SHELX 2016.25 Hydrogen atoms were located in a difference map, added geometrically, and refined with a riding model. The data can be obtained free of charge from The Cambridge Crystallographic Data Centre with CCDC no. 1547067. |
| ¶ PBE/TZ2P level, Priroda code.26 |
| This journal is © The Royal Society of Chemistry 2017 |