Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The efficient synthesis and purification of amyloid-β(1–42) using an oligoethylene glycol-containing photocleavable lysine tag†
John A.
Karas
 *ab,
Asif
Noor
a,
Christine
Schieber
a,
Timothy U.
Connell
a,
Frances
Separovic
*ab,
Asif
Noor
a,
Christine
Schieber
a,
Timothy U.
Connell
a,
Frances
Separovic
 a and
Paul S.
Donnelly
a and
Paul S.
Donnelly
 *a
*a
aSchool of Chemistry and Bio21 Molecular Science and Biotechnology Institute, The University of Melbourne, VIC 3010, Australia. E-mail: jkaras@unimelb.edu.au; pauld@unimelb.edu.au
bDepartment of Chemical and Biomolecular Engineering, The University of Melbourne, VIC 3010, Australia
First published on 6th June 2017
Abstract
A short, monodisperse oligoethylene glycol-containing photocleavable lysine tag was developed to facilitate the efficient purification of hydrophobic and fibril-forming peptides. This new tag was used to prepare a modified Aβ42 peptide with increased solubility and decreased propensity to aggregate in aqueous media. The solubilising tag was readily removed by irradiation with UV light and permitted the preparation and isolation of Aβ42 in high purity and yield.
Alzheimer's disease is the most common form of neurodegenerative dementia. Hallmarks of the disease include extracellular plaques and intracellular tau-enriched neurofibrillary tangles in the brain.1,2 The plaques contain aggregated forms of the amyloid-β (Aβ) polypeptide ranging in length from 39–43 amino acid residues, formed by proteolytic cleavage of amyloid precursor protein. The two main peptides produced are 40 (Aβ40) and 42 (Aβ42) amino acid residues in length. The longer Aβ42 has a greater propensity for aggregation in vivo and is often considered the more toxic.3 Continued efforts to better understand the structure of Aβ42 in fibrils and plaques,4–6 the toxicity of Aβ42 oligomers and assessing potential therapeutic molecules7 all require ready access to pure synthetic Aβ42.
Solid-phase peptide synthesis (Fmoc SPPS, Fmoc = 9-fluorenylmethoxycarbonyl) can be used to prepare Aβ42.8 Unfortunately, isolation of Aβ42 using conventional chromatographic techniques is extremely challenging. The propensity of Aβ42 to aggregate results in broad and asymmetrical peaks when attempting to purify the crude peptide by high performance liquid chromatography (HPLC). This makes fractionation of the target peptide from closely-eluting peptidic impurities difficult.9
Several strategies have been employed to improve chromatographic peak resolution and solubility of ‘difficult peptides’,9 including the use of alkaline buffers to alter the net charge of the peptide10 and high temperature HPLC.11 Temporary chemical modification of hydrophobic peptides with iso-acyl dipeptides12 and polycationic orthogonally cleavable tags also possess effective solubilising properties in acidic media, which is typically used during RP-HPLC purifications.13–17 The addition of PEGylated or OEGylated (PEG = polyethylene glycol, OEG = oligoethylene glycol) functional groups can increase aqueous solubility over a wide pH range without increasing ionic charge and both are simple to incorporate during Fmoc SPPS.18 It has been shown that ortho-nitrobenzyl (oNb) based photocleavable protecting groups are compatible with SPPS.19–21 In this manuscript we describe a new amine-reactive, photolabile OEG tag that enhances the solubility of Aβ42 and enables its more efficient preparation.
Amine-reactive poly-disperse PEG reagents have been employed to switch on the function of proteins with light, but a small monodisperse OEG-containing substrate was considered more appropriate in this instance since a poly-disperse product would be difficult to isolate.22 It was thought that this approach may also allow some control of fibril formation.23
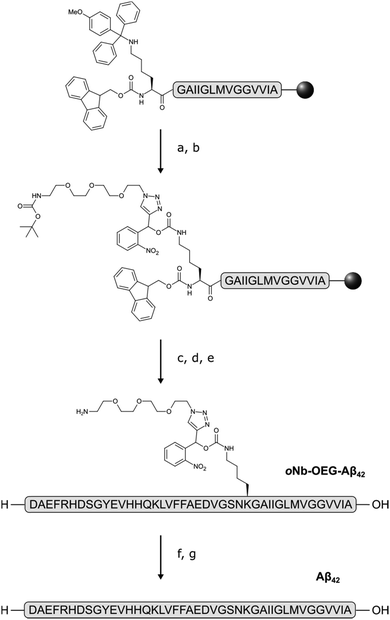
An oNb-based substrate containing a tert-butyloxycarbonyl (Boc) protected amino-OEG3 chain (oNb-OEG3) was synthesised by adapting a method of Rossi and co-workers (Scheme 1).24 Incorporation of an alkyne functional group at the benzylic position of the oNb derivative via a Grignard reaction gave 1.17 Copper(I)-catalyzed azide–alkyne cyclo-addition (CuAAC) between 1 and 2 gave 3 in a yield of 72%, that was then reacted with para-nitrophenyl chloroformate to produce 4 in 45% yield.
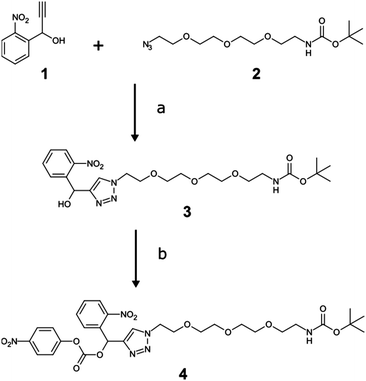 | ||
| Scheme 1 Synthesis of the oNb-OEG3 tag. (a) CuSO4, sodium ascorbate, DMF/water; (b) para-nitrophenyl chloroformate, DCM. | ||
Although it would be simplest to incorporate the hydrophilic tag at the N-terminus, we chose to functionalise Lys28 due to its close proximity to the hydrophobic C-terminus that is essential for aggregation. Residues 28–42 of Aβ42 were assembled on the solid support by microwave-assisted Fmoc SPPS, with mono-methoxytrityl (Mmt) protection at Lys28 (Scheme 2). The ε-amino group was liberated after multiple treatments with dilute trifluoroacetic acid (TFA), followed by acylation with para-nitrophenylcarbamate activated oNb-OEG3 at high temperature. The remainder of the peptide was assembled by SPPS, followed by global deprotection and cleavage from the solid support.
Electrospray ionisation mass spectrometry (ESI-MS) (see ESI†) and analytical RP-HPLC (Fig. 1a) indicated that the target peptide was the major species present in the crude reaction mixture. The purification conditions which gave optimal peak separation were alkaline buffers at 60 °C using a C18 column. It is very difficult to analyze or purify Aβ42 with a C18 stationary phase so its use in this instance suggests that the oNb-OEG3 tag does reduce the peptide's propensity to aggregate. The presence of the solubilising tag allowed isolation of oNb-OEG3-Aβ42 in excellent yield (10%) and high purity (Fig. 1b). The solubilising OEG-tag could be readily removed by irradiation at 365 nm (20 min) (Fig. 2). The photolysis was performed in 80% aqueous 1,1,1,3,3,3-hexa-fluoroisopropanol (HFIP) to avoid premature aggregation prior to final purification. A shift in retention time was observed post-cleavage (Fig. 1c) as well as significant peak broadening, which is characteristic of native Aβ42. Importantly, no significant degradation caused by the photolysis was evident and the elution profile of the target material was well resolved from non-peptidic species originating from the oNb-OEG3 tag.
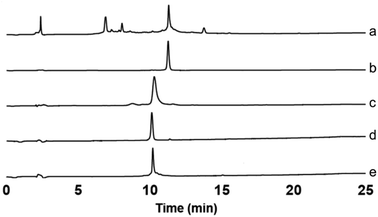 | ||
| Fig. 1 Analytical RP-HPLC traces. (a) Crude oNb-OEG3-Aβ42; (b) purified oNb-OEG3-Aβ42; (c) crude (post-photolysis) Aβ42; (d) purified (post-photolysis) Aβ42; (e) control Aβ42. | ||
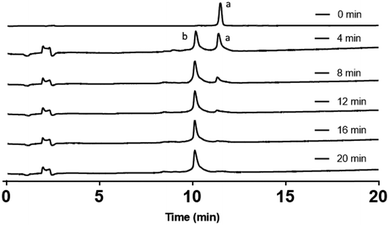 | ||
| Fig. 2 Analytical RP-HPLC traces of a time course for the photolysis reaction. Peak (a) oNb-OEG-Aβ42; peak (b) Aβ42 obtained after photolysis of oNb-OEG-Aβ42. | ||
The Aβ42 peptide was purified and isolated in good yield and high purity (Fig. 1d) using semi-preparative RP-HPLC on a C4 column. The identity of the final product was confirmed by ESI-MS (see ESI†). The isolated yield from the photolysis reaction was 60% after a single HPLC purification.
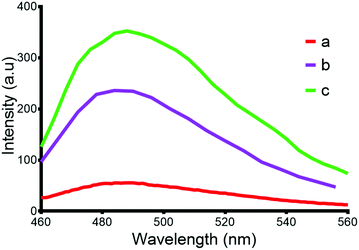
The presence of amyloid fibrils is often determined by monitoring the fluorescence of thioflavin-T (2-[p-(dimethyl-amino)phenyl]-3,6-dimethyl-benzothiazolium chloride, ThT), which undergoes a 115 nm red shift in its excitation and emission profile following interaction with amyloid fibrils. The presence of the solubilising tag in Aβ42 results in less fluorescence from ThT after incubation for 48 hours when compared to a sample of native Aβ42 prepared from the photolysis of oNb-OEG-Aβ42 (Fig. 3). This is presumably due to the tag hindering the formation of amyloid fibrils. The structural morphology of the aggregates after photolysis of oNb-OEG-Aβ42 was investigated by transmission electron microscopy (TEM), and confirmed the formation of Aβ42 fibrils (Fig. 4b). In contrast, the TEM image of oNb-OEG-Aβ42 does not show well defined fibril structures characteristic of native peptide aggregates (Fig. 4a). Given these results, it appears that strategic placement of the oNb-OEG3 tag is effective in suppressing amyloid fibril formation. This could explain why oNb-OEG-Aβ42 was obtained at such a high purity.
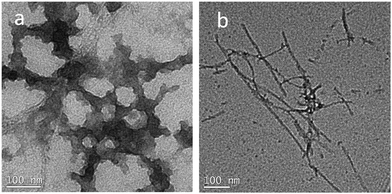 | ||
| Fig. 4 TEM images after incubation for 48 hours at 37 °C in PBS, negatively stained with 2% uranyl acetate (a) oNb-OEG-Aβ42 (10 μM); and (b) Aβ42 (10 μM) prepared by photolysis of oNb-OEG-Aβ42. | ||
In conclusion, a photocleavable hydrophilic tag, oNb-OEG, has been prepared and incorporated into Aβ42 at Lys28 using Fmoc SPPS. The presence of the tag improved the solubility of this hydrophobic peptide in aqueous media and suppressed the formation of aggregates and fibrils. The increased solubility enabled the synthesis and isolation of oNb-OEG-Aβ42 in good yield and high purity. The tag could be readily removed by photolysis. It is likely that this strategy could be used for the synthesis of other hydrophobic and fibril-forming “difficult” peptides.9
We would like to acknowledge the Australian Research Council for financial support of this research.
References
- J. Hardy and D. Allsop, Trends Pharmacol. Sci., 1991, 12, 383–388 CrossRef CAS PubMed.
- E. Jamasbi, J. D. Wade, F. Separovic and M. A. Hossain, Curr. Med. Chem., 2016, 23, 884–892 CrossRef CAS PubMed.
- M. Citron, Nat. Rev. Drug Discovery, 2010, 9, 387–398 CrossRef CAS PubMed.
- M. T. Colvin, R. Silvers, Q. Z. Ni, T. V. Can, I. Sergeyev, M. Rosay, K. J. Donovan, B. Michael, J. Wall, S. Linse and R. G. Griffin, J. Am. Chem. Soc., 2016, 138, 9663–9674 CrossRef CAS PubMed.
- N. J. Economou, M. J. Giammona, T. D. Do, X. Zheng, D. B. Teplow, S. K. Buratto and M. T. Bowers, J. Am. Chem. Soc., 2016, 138, 1772–1775 CrossRef CAS PubMed.
- Y. Xiao, B. Ma, D. McElheny, S. Parthasarathy, F. Long, M. Hoshi, R. Nussinov and Y. Ishii, Nat. Struct. Mol. Biol., 2015, 22, 499–505 CAS.
- D. J. Hayne, S. Lim and P. S. Donnelly, Chem. Soc. Rev., 2014, 43, 6701–6715 RSC.
- E. Atherton, C. J. Logan and R. C. Sheppard, J. Chem. Soc., Perkin Trans. 1, 1981, 538–546 RSC.
- M. Paradís-Bas, J. Tulla-Puche and F. Albericio, Chem. Soc. Rev., 2015, 45, 631–654 RSC.
- W. M. Kok, J. M. Cottam, G. D. Ciccotosto, L. A. Miles, J. A. Karas, D. B. Scanlon, B. R. Roberts, M. W. Parker, R. Cappai, K. J. Barnham and C. A. Hutton, Chem. Sci., 2013, 4, 4449 RSC.
- G. Vanhoenacker and P. Sandra, Anal. Bioanal. Chem., 2008, 390, 245–248 CrossRef CAS PubMed.
- T. Yoshiya, A. Taniguchi, Y. Sohma, F. Fukao, S. Nakamura, N. Abe, N. Ito, M. Skwarczynski, T. Kimura, Y. Hayashi and Y. Kiso, Org. Biomol. Chem., 2007, 5, 1720 CAS.
- M. A. Hossain, A. Belgi, F. Lin, S. Zhang, F. Shabanpoor, L. Chan, C. Belyea, H.-T. Truong, A. R. Blair, S. Andrikopoulos, G. W. Tregear and J. D. Wade, Bioconjugate Chem., 2009, 20, 1390–1396 CrossRef CAS PubMed.
- J.-S. S. Zheng, Y. He, C. Zuo, X.-Y. Y. Cai, S. Tang, Z. A. Wang, L.-H. H. Zhang, C.-L. L. Tian and L. Liu, J. Am. Chem. Soc., 2016, 138, 3553–3561 CrossRef CAS PubMed.
- M. T. Jacobsen, M. E. Petersen, X. Ye, M. Galibert, G. H. Lorimer, V. Aucagne and M. S. Kay, J. Am. Chem. Soc., 2016, 138, 11775–11782 CrossRef CAS PubMed.
- C. Zuo, S. Tang, Y.-Y. Y. Si, Z. A. Wang, C.-L. L. Tian and J.-S. S. Zheng, Org. Biomol. Chem., 2016, 14, 5012–5018 CAS.
- S. Chemuru, R. Kodali and R. Wetzel, Biopolymers, 2014, 102, 206–221 CrossRef CAS PubMed.
- R. V. Pillai and M. Mutter, Acc. Chem. Res., 1981, 14, 122–130 CrossRef.
- P. Klán, T. Šolomek, C. G. Bochet, A. Blanc, R. Givens, M. Rubina, V. Popik, A. Kostikov and J. Wirz, Chem. Rev., 2013, 113, 119–191 CrossRef PubMed.
- N. Kotzur, B. Briand, M. Beyermann and V. Hagen, J. Am. Chem. Soc., 2009, 131, 16927–16931 CrossRef CAS PubMed.
- J. A. Karas, D. B. Scanlon, B. E. Forbes, I. Vetter, R. J. Lewis, J. Gardiner, F. Separovic, J. D. Wade and M. A. Hossain, Chem. – Eur. J., 2014, 20, 9549–9552 CrossRef CAS PubMed.
- W. E. Georgianna, H. Lusic, M. L. Andrew and A. Deiters, Bioconjugate Chem., 2010, 21, 1404–1407 CrossRef CAS PubMed.
- Y. Sohma, Y. Hayashi, M. Kimura, Y. Chiyomori, A. Taniguchi, M. Sasaki, T. Kimura and Y. Kiso, J. Pept. Sci., 2005, 11, 441–451 CrossRef CAS PubMed.
- J. A. Baccile, M. A. Morrell, R. M. Falotico, B. T. Milliken, D. L. Drew and F. M. Rossi, Tetrahedron Lett., 2012, 53, 1933–1935 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details of organic and peptide synthesis, purification, photolysis and aggregation studies. See DOI: 10.1039/c7cc03147e |
| This journal is © The Royal Society of Chemistry 2017 |