Open Access Article
Open Access ArticleThe first molecular dumbbell consisting of an endohedral Sc3N@C80 and an empty C60-fullerene building block†
Tao
Wei‡
,
M. Eugenia
Pérez-Ojeda‡
 and
Andreas
Hirsch
and
Andreas
Hirsch
 *
*
Department of Chemistry and Pharmacy and Joint Institute of Advanced Materials and Processes (ZMP), Friedrich-Alexander University Erlangen-Nuremberg, Henkestrasse 42, D-91054 Erlangen, Germany. E-mail: andreas.hirsch@chemie.uni-erlangen.de; Fax: +49 9131 85 26864; Tel: +49 9131 85 22537
First published on 19th May 2017
Abstract
An unprecedented hybrid dumbbell consisting of a metallofullerene and an empty fullerene was afforded via simple click reaction of suitable precursor derivatives of Sc3N@C80 and a C60 hexakisadduct.
Dumbbell shaped fullerenes represent an interesting class of compounds. Their molecular architectures have been attracting great attention due to their distinct chemical/physical properties.1 To date, various series of such dimers based on the introduction of different types of fullerenes (e.g. C60, C70, heterofullerenes, endofullerenes) as well as various linkers serving as bridges have been reported (see Fig. 1).2–11 One striking characteristic of dimeric fullerene dumbbells is their tunable properties by the possibility of modifying the chemical nature of fullerene building blocks or the chemical linkage between them. So far however, only either empty fullerenes, heterofullerenes (C59N) or the non-metallic endohedral fullerenes such as He@C60, H2@C60 and N@C602 have been employed where the corresponding molecular architectures containing endohedral clusterfullerenes are elusive. Among these examples and due to their unique high spin behavior, dimers based on N@C60 promise feasibility for quantum computer applications.2 However, the incorporation of endohedral clusterfullerenes, especially metal nitride cluster fullerenes (NCFs), is still lacking. In these endofullerene derivatives the encapsulated cluster comprising of both metal and non-metal ions gives rise to an electron transfer between the endohedral guest and the carbon cage. This feature which is absent in empty fullerenes or N@C60 makes the synthesis of dumbbells an interesting task since the properties of both kinds of fullerenes can be combined in one molecule. The only cases of dimeric endohedral metallofullerenes were reported to take place in the solid state after crystallization.12–14
Our group has previously demonstrated the versatility of [5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0] pentakisadducts of C60 with C2v symmetry as useful building blocks for the synthesis of large and highly functional architectures involving an octahedral [5
0] pentakisadducts of C60 with C2v symmetry as useful building blocks for the synthesis of large and highly functional architectures involving an octahedral [5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] addition pattern.15 For the targeted synthesis of the first dumbbell consisting of an endohedral and an empty fullerene we decided to prepare a [5
1] addition pattern.15 For the targeted synthesis of the first dumbbell consisting of an endohedral and an empty fullerene we decided to prepare a [5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
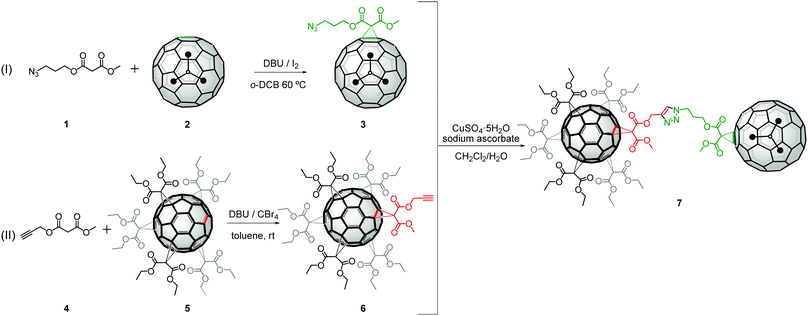
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1]-adduct of C60 equipped with 10 chains which will facilitate the solubility of the dumbbell (aliphatic ones in this case) and one terminal alkyne group. This should serve as an anchor unit for the “click” coupling with a monoadduct of the endohedral clusterfullerene-Sc3N@Ih-C80 containing the complementary azide functionality in its periphery.
1]-adduct of C60 equipped with 10 chains which will facilitate the solubility of the dumbbell (aliphatic ones in this case) and one terminal alkyne group. This should serve as an anchor unit for the “click” coupling with a monoadduct of the endohedral clusterfullerene-Sc3N@Ih-C80 containing the complementary azide functionality in its periphery.
Sc3N@Ih-C80 was chosen as an endohedral fullerene building block since it is easily available in rather high yields.16 On the other hand, its high cage symmetry bearing two available reaction positions allows the formation of only two possible regioisomeric monoadducts. Altogether the synthesis of the targeted fullerene dumbbell consists of three steps: (1) the synthesis of an azidomalonate monoadduct of Sc3N@Ih-C80; (2) the selective synthesis of a [5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] hexakisadduct of C60 provided with an alkyne and (3) the covalent connection of these subunits via “click” reaction.
1] hexakisadduct of C60 provided with an alkyne and (3) the covalent connection of these subunits via “click” reaction.
In the first step, we aimed to synthesize an azidomalonate monoadduct of Sc3N@Ih-C80 using Bingel–Hirsch reaction conditions (Scheme 1 (I) and for further details see S2.1, ESI†). The subsequent purification afforded a pure major product (denoted as 3) which was confirmed by matrix-assisted laser desorption time-of-flight (MALDI-TOF) mass spectrometry analysis showing an ionic peak at m/z = 1308 (Fig. S6, ESI†).
Additionally, both 1H and 13C NMR were performed to unambiguously elucidate its structure. The 1H NMR spectrum of 3 exhibits one set of signals featuring one quartet, one singlet and two triplets centered at 4.59, 4.11, 3.50 and 2.09 ppm, respectively (Fig. S7, ESI†). The singlet at 3.50 ppm corresponds to the methoxy group and the other three resonances can be attributed to protons of the three remaining methylene groups. A potential [5,6]-adduct would give rise to two sets of nonequivalent protons as a result of different chemical environments stemming from the 5,6-ring junction, whereas the more symmetrical [6,6]-adduct gives only one set of proton signals. The fact that just one symmetrical [6,6]-adduct was formed is in good agreement with previous studies on M3N@Ih-C80 adducts synthesized via Bingel–Hirsch reactions.17 The 13C NMR spectrum of 3 shows 5 signals in the range of 20–60 ppm and two additional signals around 163 ppm for the attached group, besides, another 80 resonances distributed in the 85–155 ppm region were observed for the carbon cage, consistent with C1 symmetry (Fig. S8, ESI†). The C1 symmetry caused by addition of an asymmetric functionalized moiety is in line with the previously reported Sc3N@C80–PCBM, in which the C1 symmetry was clearly confirmed by both 13C NMR and X-ray crystallographic studies.18
In the second step, the [5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] hexakisadduct of C60 (denoted as 6) was facilely synthesized by Bingel–Hirsch reactions (Scheme 1 (II) and for further details see S2.2, ESI†). Finally, in order to construct the target dumbbell, a copper-catalyzed Huisgen 1,3-dipolar cycloaddition between azides and alkynes “click” reaction was conducted by using 3 and 1.5-fold of 6 under modified Sharpless conditions at room temperature with CuSO4·5H2O (0.3 equiv.) and sodium ascorbate (0.9 equiv.) in a CH2Cl2/H2O biphasic system, under a nitrogen atmosphere for 96 h (Scheme 1). Upon such a mild and long reaction treatment the assumed 1,2,3-triazole-linked dumbbell comprising of 3 and 6 was formed. Surprisingly, despite the presence of a Cu(I) catalyst, the clicked product afforded was not the expected sole 1,4-adduct but an isomeric mixture in a 3
1] hexakisadduct of C60 (denoted as 6) was facilely synthesized by Bingel–Hirsch reactions (Scheme 1 (II) and for further details see S2.2, ESI†). Finally, in order to construct the target dumbbell, a copper-catalyzed Huisgen 1,3-dipolar cycloaddition between azides and alkynes “click” reaction was conducted by using 3 and 1.5-fold of 6 under modified Sharpless conditions at room temperature with CuSO4·5H2O (0.3 equiv.) and sodium ascorbate (0.9 equiv.) in a CH2Cl2/H2O biphasic system, under a nitrogen atmosphere for 96 h (Scheme 1). Upon such a mild and long reaction treatment the assumed 1,2,3-triazole-linked dumbbell comprising of 3 and 6 was formed. Surprisingly, despite the presence of a Cu(I) catalyst, the clicked product afforded was not the expected sole 1,4-adduct but an isomeric mixture in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 1,4 and a 1,5-adducts which would have been likely under thermal conditions. This interesting observation is indicative of some not yet well understood effects of copper on the Sc3N@Ih-C80 properties, charge transfer or redox activity.19 On account of a very similar polarity of these two isomers, their complete separation is quite challenging. Fortunately, a partial pure major isomer (denoted as 7) was successfully isolated on a silica-gel chromatographic column thanks to the good solubility provided by the linkage to the hexakisadduct of C60. The yield of the major isomer of dumbbell-7, was roughly estimated to be 45% regardless of its small quantity that could not be isolated from the mixture of isomers.
1 ratio of 1,4 and a 1,5-adducts which would have been likely under thermal conditions. This interesting observation is indicative of some not yet well understood effects of copper on the Sc3N@Ih-C80 properties, charge transfer or redox activity.19 On account of a very similar polarity of these two isomers, their complete separation is quite challenging. Fortunately, a partial pure major isomer (denoted as 7) was successfully isolated on a silica-gel chromatographic column thanks to the good solubility provided by the linkage to the hexakisadduct of C60. The yield of the major isomer of dumbbell-7, was roughly estimated to be 45% regardless of its small quantity that could not be isolated from the mixture of isomers.
The purified product enables characterization to confirm its molecular structure as well as its electronic properties. Given that mass spectrometry can provide the most intuitive information about the composition of the molecule, it was immediately applied to 7. As shown in Fig. 2, the MALDI-TOF mass spectrum of dumbbell-7 exhibited an evident ionic peak at m/z = 2972 corresponding to the clicked products of 3 and 6. The chemical identification of dumbbell-7 was further achieved by comparing the isotopic distribution analysis of the experimental measured result with the calculated one since a good agreement is observed. Consequently, for the first time, an unprecedented dumbbell based on endohedral clusterfullerenes was successfully achieved via chemical modification.
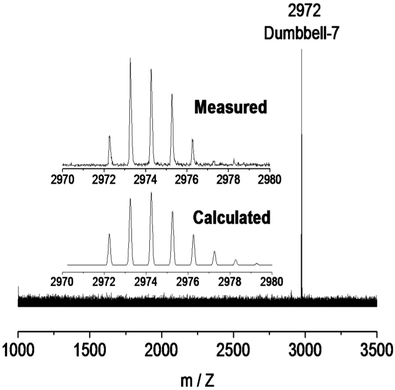 | ||
| Fig. 2 MALDI-TOF spectrum of dumbbell-7. The inset shows the measured and calculated isotope distributions. | ||
The formation of the dumbbell was further confirmed by the 1H NMR spectroscopy study as shown in Fig. S19 (ESI†). In the 1H NMR spectrum of dumbbell-7, the four resonances centered at 4.52, 4.12, 3.57 and 2.29 ppm are obviously derived from monomer 3 and the other three resonances centered at 4.34, 3.80, and 1.31 ppm clearly originated from monomer 6. Interestingly, all these resonances of dumbbell-7 are either low field or high field shifted compared to two monomers, suggesting that linking these two building blocks for the dumbbell formation brings about changes in the chemical environment. Noticeably, a singlet resonance centered at 7.71 ppm corresponding to the proton situated at 1,2,3-triazole as a result of the click reaction between the azido group within 3 and the alkynyl group within 6, clearly states that the formation of the dumbbell has been successfully proceeded. Unfortunately, the limited amount of dumbbell-7 precluded the acquisition of its 13C NMR spectrum.
The electronic properties of dumbbell-7 were investigated by UV-vis-NIR spectroscopy. The UV-vis-NIR spectrum of dumbbell-7 dissolved in toluene is shown in Fig. 3, which includes also those of monomers 3 and 6 for comparison. Apparently, the overall electronic absorption spectrum of 3 is featureless without sharp absorption peaks and exhibits two broad shoulder peaks at about 440 and 748 nm, which is less rich in characteristics than that of 6, for which two obvious absorption peaks are observed at 316 and 334 nm together with a broad peak at 385 nm. For dumbbell-7, several characteristic absorption peaks, which are derived from the two building blocks, at 319, 336, 417 and 737 nm, are clearly observed. Specifically, two sharp absorption peaks, at 319 and 336 nm, in the UV region apparently originated from one of the individuals of 6, whereas the other individual of 3 accounts for the remaining two absorption peaks in the visible region. Besides, the absorption peaks at 319 and 336 nm of dumbbell-7 seem to be red-shifted compared to those of 6, in contrast, a blue-shift for the other two peaks, at 417 and 737 nm, is detected in comparison with that of 3. Regardless of the slight red/blue shift, the overall absorption spectrum of dumbbell-7 is quite similar to the superposition spectra of the two monomers, indicating that dumbbell-7 inherits the electronic properties of the two constituents. Therefore this methodology opens up the possibility of combining the electronic properties of two fullerenes with deeply different natures. By a careful selection of the appropriate parental counterparts, the dumbbell formation might be a very versatile tool to tune the electronic properties as demanded by the applications. It will allow the synthesis of novel fullerene derivatives with attractive properties which may diversify the potential applications of fullerene-based multifunctional molecules.
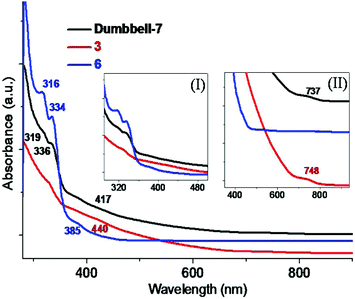 | ||
| Fig. 3 UV-vis-NIR spectrum of dumbbell-7 dissolved in toluene in comparison with those of 3 and 6. Insets: Enlarged spectra in 300–500 nm (I) and 350–950 nm (II) regions. | ||
Besides, in order to elucidate which is the real influence of the endofullerene, dumbbell-7 was further compared to congener dimer-9, which was synthesized by substitution of the azido monoadduct of Sc3N@C80 with the azido monoadduct of C60 (8) (for details see S3, ESI†). Under the same experimental conditions, only 1,4-adduct formation was observed which clearly indicates the influence of the endofullerene in the copper catalyzed click mechanism as explained above. As expected, the UV-vis-NIR spectrum of dimer-9 bears a resemblance to the overlap of the absorption peaks from the corresponding monomers 8 and 6 (Fig. S20, ESI†).
Moreover, noting the existing six charges within 3 as a result of charge transfer from encaged species to the carbon cage, the investigation on the probable charge transfer between these two building blocks is convinced to be quite attractive, and will be carried out in the future.
In summary, we have synthesized and isolated the hitherto unknown dumbbell-7via bonding of the endohedral clusterfullerene monodadduct 3 with the C60 hexakisadduct 6. The structure of synthetic dumbbell-7 was unambiguously characterized by MALDI-TOF mass spectrometry and 1H NMR spectroscopy. The electronic structure was carefully studied by UV-vis-NIR spectroscopy and compared to parental building blocks and congener dimer-9, and the peculiar electronic properties achieved by a combination of two different moieties in the dumbbell are unveiled. Our success in the first construction of an endohedral clusterfullerene dumbbell opens up a new perspective in fullerene chemistry.
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) through SFB 953, the Alexander von Humboldt (AvH) Foundation (Tao Wei) and Marie Sklodowska-Curie IF European Actions 747734 Hy-solFullGraph to M. E. Pérez-Ojeda. The authors want to acknowledge Dr. Frank Hauke for the cover design.
Notes and references
- For recent typical reviews (a) F. Giacalone and N. Matiín, Chem. Rev., 2006, 106, 5136–5190 CrossRef CAS PubMed; (b) A. L. Balch and K. Winkler, Chem. Rev., 2016, 116, 3812–3882 CrossRef CAS PubMed.
- B. J. Farrington, M. Jevric, G. A. Rance, A. Ardavan, A. N. Khlobystov, G. A. D. Briggs and K. Porfyrakis, Angew. Chem., Int. Ed., 2012, 124, 3647–3650 CrossRef.
- K. Komatsu, K. Fujiwara and Y. Murata, Chem. Commun., 2000, 1583–1584 RSC.
- G. S. Forman, N. Tagmaatarchis and H. Shinohara, J. Am. Chem. Soc., 2002, 124, 178–179 CrossRef CAS PubMed.
- J. C. Hummelen and F. Wudl, Science, 1995, 269, 1554–1556 CAS.
- F. Hauke, M. Á. Herranz, L. Echegoyen, D. Guldi, A. Hirsch and S. Atalick, Chem. Commun., 2004, 600–601 RSC.
- B. Nuber and A. Hirsch, Chem. Commun., 1996, 1421–1422 RSC.
- F. Hörmann, A. Hirsch, K. Porfyrakis and G. A. D. Briggs, Eur. J. Org. Chem., 2011, 117–121 CrossRef.
- K. Komatsu, G. W. Wang, Y. Murata, T. Tanaka, K. Fujiwara, K. Yamamoto and M. Saunders, J. Org. Chem., 1998, 63, 9358–9366 CrossRef CAS.
- M. Murata, Y. Murata and K. Komatsu, J. Am. Chem. Soc., 2006, 128, 8024–8033 CrossRef CAS PubMed.
- G. W. Wang, K. Komatsu, Y. Murata and M. Shiro, Nature, 1997, 387, 583–586 CrossRef CAS.
- L. Bao, C. Pan, Z. Slanina, F. Uhlik, T. Akasaka and X. Lu, Angew. Chem., Int. Ed., 2016, 55, 9234–9238 CrossRef CAS PubMed.
- L. Feng, T. Tsuchiya, T. Wakahara, T. Nakahodo, Q. Piao, Y. Maeda, T. Akasaka, T. Kato, K. Yoza, E. Horn, N. Mizorogi and S. Nagase, J. Am. Chem. Soc., 2006, 128, 5990–5991 CrossRef CAS PubMed.
- D. Konarev, L. V. Zorina, S. Khasanov, A. A. Popov, A. Otsuka, H. Yamochi, G. Saito and R. N. Lyubovskaya, Chem. Commun., 2016, 52, 10763–10766 RSC.
- (a) F. Hörmann, W. Donaubauer, F. Hampel and A. Hirsch, Chem. – Eur. J., 2012, 18, 3329–3337 CrossRef PubMed; (b) L. K. Wasserthal, A. Kratzer and A. Hirsch, Eur. J. Org. Chem., 2013, 2355–2361 CrossRef CAS; (c) F. Hörmann and A. Hirsch, Chem. – Eur. J., 2013, 19, 3188–3197 CrossRef PubMed; (d) L. K. Wasserthal, B. Shade, K. Ludwig, C. Böttcher and A. Hirsch, Chem. – Eur. J., 2014, 20, 5961–5966 CrossRef CAS PubMed; (e) X. Yu, W. B. Zhang, K. Yue, X. Li, H. Liu, Y. Xin, C. L. Wang, C. Wesdemiotis and S. Z. D. Cheng, J. Am. Chem. Soc., 2012, 134, 7780–7787 CrossRef CAS PubMed; (f) J. Iehl and J. F. Nierengarten, Chem. – Eur. J., 2009, 15, 7306–7309 CrossRef CAS PubMed; (g) J. Iehl, J. F. Nierengarten, A. Harriman, T. Bura and R. Ziessel, J. Am. Chem. Soc., 2012, 134, 988–998 CrossRef CAS PubMed; (h) P. Fortgang, E. Maisonhaute, C. Amatore, B. Delavaux-Nicot, J. Iehl and J. F. Nierengarten, Angew. Chem., Int. Ed., 2011, 50, 2364–2367 CrossRef CAS PubMed; (i) J. Iehl and J. F. Nierengarten, Chem. Commun., 2010, 46, 4160–4162 RSC.
- S. Stevenson, G. Rice, T. Glass, K. Harich, F. Cromer, M. R. Jordan, J. Craft, E. Hadju, R. Bible, M. M. Olmstead, K. Maitra, A. J. Fisher, A. L. Balch and H. C. Dorn, Nature, 1999, 401, 55–57 CrossRef CAS.
- (a) C. M. Cardona, A. Kitaygorodskiy and L. Echegoyen, J. Am. Chem. Soc., 2005, 127, 10448–10453 CrossRef CAS PubMed; (b) C. Y. Shu, T. Cai, L. S. Xu, T. M. Zuo, J. Reid, K. Harich, H. C. Dorn and H. W. Gibson, J. Am. Chem. Soc., 2007, 129, 15710–15717 CrossRef CAS PubMed; (c) O. Lukoyanova, C. M. Cardona, J. Rivera, L. Z. Lugo-Morales, C. J. Chancellor, M. M. Olmstead, A. Rodríguez-Fortea, J. M. Poblet, A. L. Balch and L. Echegoyen, J. Am. Chem. Soc., 2007, 129, 10423–10430 CrossRef CAS PubMed.
- C. Y. Shu, W. Xu, C. Slebodnick, H. Champion, W. J. Fu, J. E. Reid, H. Azurmendi, C. R. Wang, K. Harich, H. C. Dorn and H. W. Gibson, Org. Lett., 2009, 11, 1753–1756 CrossRef CAS PubMed.
- During the course of the reaction it was observed that the typical yellowish color from aqueous phase containing Cu(I) species protected against oxidation in the presence of a huge excess of sodium ascorbate and an inert nitrogen atmosphere turned into blue as an evidence of some oxidative processes which could be tentatively promoted by the endofullerene moiety. Other authors have previously described that the presence of a Cu additive in the electric arc reactor dramatically affects the yield of metallofullerene Sc3N@Ih-C80, especially under amounts of Cu and Sc. However they have not yet been able to address the reason for this behaviour. S. Stevenson, M. A. Mackey, M. C. Thompson, H. L. Coumbe, P. K. Madasu, C. E. Coumbe and J. P. Phillips, Chem. Commun., 2007, 4263–4265 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental section, 1H and 13C NMR spectra, UV-vis-NIR spectrum and the MALDI-TOF mass spectrum. See DOI: 10.1039/c7cc03012f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |