Highly enantioselective synthesis of fused bicyclic dihydropyranones via low-loading N-heterocyclic carbene organocatalysis†
Abstract
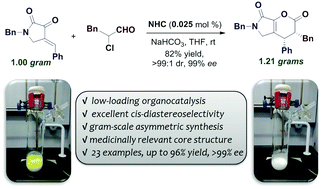
Highly diastereo and enantioselective [4+2] cycloadditions have been achieved between pyrrolidone-derived cyclic enones and α-haloaldehydes under mild conditions. Relying on extremely reactive in-situ generated chiral N-heterocyclic carbenes, this stereoselective annulation proceeds efficiently even on the gram scale with the catalyst loading as low as 0.025 mol% (250 ppm). A variety of cis-substituted bicyclic dihydropyranones can be produced in up to 96% yield with up to >99% ee. In addition, simple, inexpensive linear aldehydes such as n-propanal can be used directly in asymmetric cycloadditions via oxidative N-heterocyclic carbene organocatalysis with low catalyst loading. This method may provide an economical and practical approach for the asymmetric synthesis of medicinally relevant molecules.


 Please wait while we load your content...
Please wait while we load your content...