 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rationalizing the relative abundances of trimetallic nitride template-based endohedral metallofullerenes from aromaticity measures†
M.
Garcia-Borràs
 *ab,
S.
Osuna
*ab,
S.
Osuna
 b,
J. M.
Luis
b,
J. M.
Luis
 b and
M.
Solà
b and
M.
Solà
 *b
*b
aDepartment of Chemistry and Biochemistry, University of California, Los Angeles, California 90095, USA. E-mail: marcgbq@gmail.com
bInstitut de Química Computacional i Catàlisi (IQCC) and Departament de Química, Universitat de Girona, Campus Montilivi, 17003 Girona, Catalonia, Spain. E-mail: miquel.sola@udg.edu
First published on 21st March 2017
Abstract
The synthesis of endohedral metallofullerenes (EMFs) from a carbon soot sample of an arc discharge leads to a variety of EMFs that are obtained in different relative abundances. In the present work, we show that these abundances can be predicted from aromaticity calculations. In particular, we use the normalized Additive Local Aromaticity (ALAN) index. Our results show that the most abundant Sc3N-based and Y3N-based EMFs in fullerene soot are the most aromatic. This study reinforces the idea that aromaticity plays a key role in determining the stability of EMFs.
Endohedral metallofullerenes (EMFs) have attracted much attention since their discovery because of their extraordinary properties and potential applications.1–3 Many different EMFs are obtained by arc discharge or laser vaporization, but only a few of them are produced in macroscopic amounts. The first endohedral metallofullerene obtained in macroscopic quantities was the Sc3N@Ih-C80 trimetallic nitride template (TNT) EMF, which is the third most abundant fullerene only after C60 and C70.4 The electronic structure of EMFs can be described by the ionic model, as proposed by Poblet and coworkers.5,6 When a metallic cluster is encapsulated inside a fullerene cage, a formal charge transfer takes place from the inner moiety to the carbon structure. In the case of TNT moieties, a formal transfer of six electrons takes place; thus its electronic structure can be described as M3N6+@C2n6−. In 2007, Popov and Dunsch showed that hexaanionic empty fullerene isomers are good models for describing TNT EMF relative stabilities.7 Rodríguez-Fortea et al. demonstrated that the negative charge is mainly accumulated in the 5-membered rings (5-MRs) of the carbon structure, and that their distribution along the fullerene surface is crucial for stabilizing the final EMF.8,9 In 2015, Martín and coworkers showed that the π-energies obtained from a Hückel molecular orbital (HMO) model are useful to predict the stability of anionic and cationic fullerene isomers.10 The authors added to the calculated HMO energies an empirical correction of strain energy due to the presence of adjacent pentagon pairs (APPs). Very recently, they demonstrated that the relative isomer stability of anionic fullerenes can be predicted by counting the numbers of three types of hexagon-based motifs and the number of APPs.11
A more stable π-electronic structure is likely to be connected with a higher aromaticity. Indeed, it is well-known that the accumulation of negative charge in the 5-MRs of EMFs increases their local and global aromatic character.12 In 2013, with the goal of understanding the chemical origin of the stability of anionic fullerene cages, we reported an extensive study showing that, for a given C2n−m cage, the most stable isomers are the ones whose total aromaticity is maximized, independently of the number of APPs they have.13,14 We formulated the maximum aromaticity criterion that gave an explanation for the isolated pentagon rule (IPR) violation in EMFs. In this study, aromaticity measurements were carried out in terms of the Additive Local Aromaticity (ALA) index.13,14 We used the HOMA geometric descriptor of aromaticity to reduce the computational cost. Still, the HOMA-based calculations provided aromaticity trends similar to those obtained with more reliable, but computationally more expensive electronic multicenter indices.15
Studies of EMF stabilities performed to date analyze the relative stability of isomers of a given C2n−m cage. Herein, we have generalized the ALA index to expand its scope and allow the direct comparison of C2n−m cages of different sizes. Our aim is to understand the differences in relative abundances in the production of EMFs by arc discharge of metal/graphite rods. To achieve this goal, we propose the normalization of the ALA index per number of rings present in the fullerene structure (ALAN). Using ALAN one can compare the aromaticities of different fullerene cages regardless of the number of carbons of the fullerene structure (see eqn (1)).
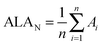 | (1) |
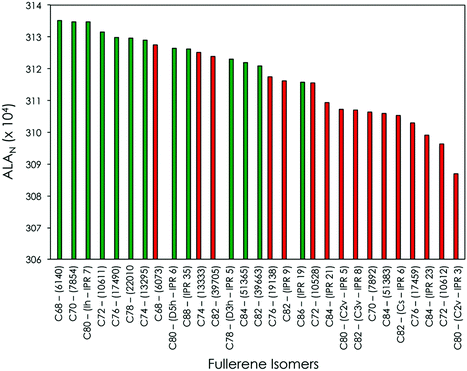 | ||
| Fig. 1 Classification in terms of the ALAN index of selected C2n6− fullerene isomers. The selection was made including the ALA top-ranked C2n6− fullerene isomers for each 2n (see ref. 13): isomers experimentally detected encapsulating TNTs or M2 clusters that formally transfer 6 electrons to the fullerene structure as well as some isomers experimentally detected encapsulating X4+ metallic clusters, such as metallic carbides. Isomers experimentally observed forming TNT EMFs are displayed in green, otherwise in red. ALAN values are given in the ALAN × 104 format. | ||
All experimentally detected TNT-containing fullerenes exhibit the largest ALAN values (on the left in Fig. 1 and represented in green), while those hexaanionic isomers that do not form TNT-based EMFs exhibit lower ALAN values (on the right in Fig. 1 and highlighted in red). It is important to mention that, in the present analysis, we consider only the three most stable (and aromatic) isomers characterized in our previous study for each C2n6− fullerene family (see ref. 13). The rest of the possible isomers present lower ALAN values and have not been experimentally observed as TNT-based EMFs. As a general trend, experimentally observed C2n6− fullerene isomers containing TNT moieties have the largest ALAN values, thus being the most aromatic ones in terms of the ALAN index. The unique exception to this rule is the isomer C82-(39705), which has an ALAN index slightly larger than the experimentally observed TNT isomer M3N@C82-(39663) (M = Gd and Y). Nevertheless, C82-(39663) is the second most aromatic C82 hexaanionic isomer, with an ALAN higher than all the other IPR and non-IPR C826− isomers. Our calculations also show that, among cages bigger than C80, C88-(IPR 5)6− is the most aromatic fullerene (see Fig. 1). Indeed, this is the preferred cage by more bulky 4f-block TNTs such as Ce3M, Pr3N or Nd3N. We can, therefore, conclude that the most aromatic C2n6− (2n = 68–88) fullerene cages, independently of their size or number of APPs, are those most suitable for stabilizing a TNT-based EMF. However, for large TNT moieties like Ce3M, Pr3N or Nd3N, predictions cannot be based only on ALAN studies because steric effects play also an important role.
Encouraged by the promising results obtained from the newly proposed ALAN index using the C2n6− model, we applied this tool for analyzing the stabilities of Sc3N@C2n (2n = 68–88) EMFs. We computed the ALAN for 10 Sc3N-based EMFs explicitly containing the scandium metallic cluster: (6140)-C68; (7854)-C70; (2210)-C78 (Y, Gd, Dy, Tm); (D3h-IPR 5)-C78; (Ih-IPR 7)-C80; (D5h-IPR 6)-C80; (39633)-C82 (Y, Gd); (51365)-C84 (Y, Gd, Tb); (D3-IPR 19)-C86 (Y, Gd, Tb); and (D2-IPR 35)-C88 (Y, Gd, Tb, Lu). In those cases where the Sc3N-based EMF has not been experimentally reported, we indicate in parentheses the metals of whose TNTs have been experimentally synthesized. The equivalent ALAN analysis for Y3N-based TNT EMFs (C78–C88) is reported in the ESI.†
In Fig. 2, the relative aromaticities of scandium-based EMFs in terms of ALAN are reported. The first clear conclusion is that Sc3N@Ih-C80, the most abundant EMF, exhibits the largest normalized aromaticity. Thus, this result suggests a direct relationship between the high stability of this compound and its high aromaticity as compared to other Sc3N-based EMFs. More importantly, the ALAN-based aromaticity tendencies nicely reproduce the relative abundances of Sc3N EMFs observed in the HPLC chromatogram directly obtained from a carbon soot sample of an arc discharge EMF synthesis. The second most abundant and thus presumably second most stable scandium TNT EMF is the D5h-C80. Then, C68 and C78 fullerenes are the third and fourth most abundant cages. An excellent agreement between the computed relative aromaticities and the relative abundances experimentally observed is therefore found. Our ALAN calculations, in agreement with the experimental HLPC measured relative abundances, also show that the Y3N@Ih-C80 isomer corresponds to the most abundant Y-based TNT EMFs (see Fig. S2–S4, ESI†). Consequently, we propose that the aromaticity is the chemical driving force that determines the relative stability of the TNT-based EMFs, and we suggest that ALAN measures can be used to predict the relative abundances of still non-synthesized EMFs.
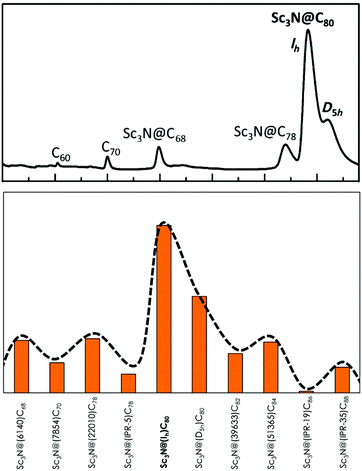 | ||
| Fig. 2 Top: Quantitative HLPC chromatograms of the arc reactor carbon sample of a typical scandium based TNT EMF synthesis (adapted with permission from a previous study by Echegoyen et al., ref. 22). Bottom: Relative ALAN scaled values ((ALAN − ALAN(min))/(ALAN(max) − ALAN(min))), being 1 for Sc3N@Ih-C80 and 0 for Sc3N@(IPR-19)-C86, of Sc3N@C2n fullerenes, sorted by increasing cage size. See the ESI† for equivalent ALAN value graphs. | ||
Minor discrepancies between experimental observations and ALAN predictions are found. For instance, the non-IPR (22010)-C78 scandium TNT EMF isomer is slightly more aromatic than the IPR D3h-C78 isomer, despite the IPR is the only experimentally observed as Sc3N@C78. The non-IPR isomer is, however, the preferred cage for encapsulating large metals such as yttrium or gadolinium.23 Our calculations for Y3N-based TNTs clearly show the Y3N preference for a non-IPR (22010)-C78 cage over an IPR D3h-C78 isomer. It should be noted that our analysis does not include thermal contributions that have been shown to be necessary to explain the formation and relative abundances of a given isomer at high temperatures.24 In addition, neither kinetic or mechanistic features nor strain effects on the TNT cluster or C2n cage are included in our predictions. These factors could explain the minor differences between the theoretically predicted EMF ratios based on their ALAN indices and those experimentally observed in an arc discharge synthesis. Nevertheless, the experimental tendency pointing to the major formation of Sc3N-based TNTs Ih-C80 and D5h-C80, followed by C68 and C78, is perfectly reproduced. This result indicates that aromaticity measures in terms of the ALAN index can be used not only to rationalize but also to predict the relative stability (in terms of abundance) of TNT EMFs.
In summary, we proposed a normalized Additive Local Aromaticity index as a measure for the relative stability of any hexaanionic fullerene C2n−6 isomer regardless of their size (from 2n = 68 to 88, a typical range for TNT EMF formation), or their number of pentagon adjacencies. We have applied our new index (ALAN) to study the relative stabilities of a series of Sc3N-based EMFs. Our results show that Sc3N@Ih-C80, the most abundant EMF, is also the most aromatic, indicating that aromaticity plays a key role in determining its large stability. Even more, the good agreement between the ALAN values obtained for the Sc3N- and Y3N-based EMF series and the experimental formation ratio indicates that this new measure can be used to understand the chemical origin of the relative stabilities of EMFs, but most importantly to predict their relative abundances.
We are grateful for financial support from the Spanish MINECO (CTQ2014-54306-P, CTQ2014-52525-P, CTQ2014-59212-P, and RyC contract to S.O.), the Catalan DIUE (2014SGR931, ICREA Academia 2014 Award to M.S. and XRQTC), and the FEDER fund (UNGI10-4E-801). M.G.-B. thanks the Spanish MECD for a PhD grant (AP2010-2517) and the Ramón Areces Foundation for a postdoctoral fellowship. S.O. thanks the European Community for the CIG project (PCIG14-GA-2013-630978), and acknowledges the funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (ERC-2015-StG-679001). CSUC and BSC-CNS are acknowledged.
Notes and references
- H. W. Kroto, J. R. Heath, S. C. Obrien, R. F. Curl and R. E. Smalley, Nature, 1985, 318, 162–163 CrossRef CAS.
- J. R. Heath, S. C. O'Brien, Q. Zhang, Y. Liu, R. F. Curl, H. W. Kroto, F. K. Tittel and R. E. Smalley, J. Am. Chem. Soc., 1985, 107, 7779–7780 CrossRef CAS.
- A. A. Popov, S. Yang and L. Dunsch, Chem. Rev., 2013, 113, 5989–6113 CrossRef CAS PubMed.
- S. Stevenson, G. Rice, T. Glass, K. Harich, F. Cromer, M. R. Jordan, J. Craft, E. Hadju, R. Bible, M. M. Olmstead, K. Maitra, A. J. Fisher, A. L. Balch and H. C. Dorn, Nature, 1999, 401, 55–57 CrossRef CAS.
- J. M. Campanera, C. Bo, M. M. Olmstead, A. L. Balch and J. M. Poblet, J. Phys. Chem. A, 2002, 106, 12356–12364 CrossRef CAS.
- J. M. Campanera, C. Bo and J. M. Poblet, Angew. Chem., Int. Ed., 2005, 44, 7230–7233 CrossRef CAS PubMed.
- A. A. Popov and L. Dunsch, J. Am. Chem. Soc., 2007, 129, 11835–11849 CrossRef CAS PubMed.
- A. Rodríguez-Fortea, N. Alegret, A. L. Balch and J. M. Poblet, Nat. Chem., 2010, 2, 955–961 CrossRef PubMed.
- A. Rodríguez-Fortea, A. L. Balch and J. M. Poblet, Chem. Soc. Rev., 2011, 40, 3551–3563 RSC.
- Y. Wang, S. Díaz-Tendero, M. Alcamí and F. Martín, Nat. Chem., 2015, 7, 927–934 CrossRef CAS PubMed.
- Y. Wang, S. Díaz-Tendero, F. Martín and M. Alcamí, J. Am. Chem. Soc., 2016, 138, 1551–1560 CrossRef CAS PubMed.
- J.-i. Aihara, Y. Nakagami and R. Sekine, J. Phys. Chem. A, 2015, 119, 6542–6550 CrossRef CAS PubMed.
- M. Garcia-Borràs, S. Osuna, M. Swart, J. M. Luis and M. Solà, Angew. Chem., Int. Ed., 2013, 52, 9275–9278 CrossRef PubMed.
- M. Garcia-Borràs, S. Osuna, J. M. Luis, M. Swart and M. Solà, Chem. Soc. Rev., 2014, 43, 5089–5105 RSC.
- F. Feixas, E. Matito, J. Poater and M. Solà, Chem. Soc. Rev., 2015, 44, 6434–6451 RSC.
- J. Cioslowski, E. Matito and M. Solà, J. Phys. Chem. A, 2007, 111, 6521–6525 CrossRef CAS PubMed.
- E. Matito, M. Duran and M. Solà, J. Chem. Phys., 2005, 122, 014109 CrossRef PubMed.
- E. Matito, M. Solà, P. Salvador and M. Duran, Faraday Discuss., 2007, 135, 325–345 RSC.
- E. Matito, ESI-3D: Electron sharing indexes program for 3D molecular space partitioning, Institut de Química Computacional i Catàlisi, Universitat de Girona, Girona, 2006 Search PubMed.
- P. Salvador and E. Ramos-Cordoba, J. Chem. Phys., 2013, 139, 071103 CrossRef PubMed.
- P. Salvador and E. Ramos-Cordoba, APOST-3D, Institut de Química Computacional i Catàlisi, Universitat de Girona, Girona, 2011 Search PubMed.
- M. R. Cerón, F. F. Li and L. Echegoyen, Chem. – Eur. J., 2013, 19, 7410–7415 CrossRef PubMed.
- A. A. Popov, M. Krause, S. Yang, J. Wong and L. Dunsch, J. Phys. Chem. B, 2007, 111, 3363–3369 CrossRef CAS PubMed.
- M. Mulet-Gas, A. Rodríguez-Fortea, L. Echegoyen and J. M. Poblet, Inorg. Chem., 2013, 52, 1954–1959 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Description of the computational methods used, Fig. S1 with Sc3N ALAN analysis, and Fig. S2 and S3 with Y3N EMF ALAN analysis. See DOI: 10.1039/c7cc01750b |
| This journal is © The Royal Society of Chemistry 2017 |
