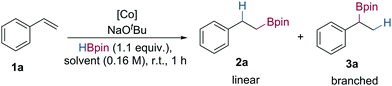Open Access Article
Open Access ArticleCobalt-catalysed Markovnikov selective hydroboration of vinylarenes†
Jingying
Peng
a,
Jamie H.
Docherty
a,
Andrew P.
Dominey
b and
Stephen P.
Thomas
 *a
*a
aEaStCHEM School of Chemistry, University of Edinburgh, David Brewster Road, Edinburgh, EH9 3FJ, UK. E-mail: stephen.thomas@ed.ac.uk
bGSK Medicines Research Centre, Gunnels Wood Road, Stevenage, SG1 2NY, Hertfordshire, UK
First published on 12th April 2017
Abstract
A bipyridiyl–oxazoline cobalt catalyst tBuBPOCoCl2 has been developed for the Markovnikov selective hydroboration of alkenes using pinacolborane and NaOtBu as the in situ activator with up to >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 branched
2 branched![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) linear selectivity (24 examples, 45–92% isolated yield).
linear selectivity (24 examples, 45–92% isolated yield).
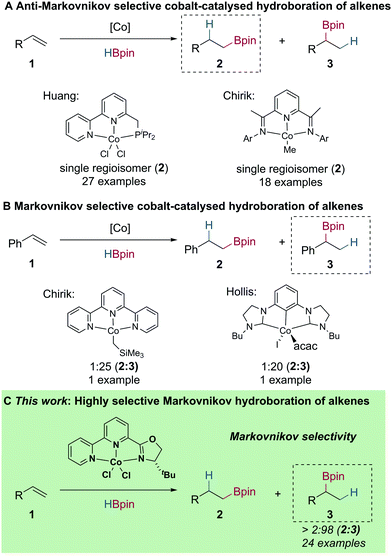
Metal-catalysed alkene hydroboration has received much attention, as the resulting boronic esters are versatile intermediates in the construction of various C–C and C–heteroatom bonds.1 The majority of metal-catalysed alkene hydroboration reactions involve complexes of precious metals, such as Rh, Ru and Ir.2 Although high chemoselectivity, regioselectivity, and enantioselectivity can be achieved, the low abundance and environmental concerns associated with these metals has driven investigations for Earth-abundant metal alternatives.3,4
In recent years, a number of Earth-abundant and base metal complexes have been developed for alkene hydroboration. In most cases, these reactions are either highly anti-Markovnikov selective or give a mixture of both Markovnikov and anti-Markovnikov products.5–18 Huang reported that bipyridyl–phosphine cobalt complexes show high activity in the anti-Markovnikov hydroboration of alkenes.5 Additionally, Chirik developed bis(imino)pyridine cobalt methyl complexes for the anti-Markovnikov selective hydroboration of alkenes (Scheme 1A).6 Of direct significance to this manuscript, Chirik7 and Hollis8 have both reported unique cobalt catalysts capable of catalysing the Markovnikov selective hydroboration of styrene in 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 20
1 and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 branched to linear ratio, respectively (Scheme 1B). Isomerisation–hydroboration using cobalt catalysis has also been reported by Chirik; which serves as an orthogonal procedure for the generation of branched boronic esters from aliphatic internal alkenes.9 However, a general reaction protocol with broad scope using a cobalt catalyst for the direct Markovnikov selective hydroboration of alkenes has not been disclosed.
1 branched to linear ratio, respectively (Scheme 1B). Isomerisation–hydroboration using cobalt catalysis has also been reported by Chirik; which serves as an orthogonal procedure for the generation of branched boronic esters from aliphatic internal alkenes.9 However, a general reaction protocol with broad scope using a cobalt catalyst for the direct Markovnikov selective hydroboration of alkenes has not been disclosed.
Herein, we report the preparation of bipyridiyl-oxazoline cobalt(II) complexes and the application of these in cobalt-catalysed alkene hydroboration using NaOtBu as the in situ pre-catalyst activator.18 This system presents a new and unique method for the generation of secondary boronic esters from readily available vinylarene starting materials with high regioselectivity, which is divergent from the majority of cobalt-catalysed alkene hydroboration reactions (Scheme 1C).
A series of cobalt complexes 5a–5c bearing novel bipyridiyl–oxazoline ligands was synthesised in four steps (Scheme 2). Reaction of 2,2′-bipyridine with hydrogen peroxide and trifluoroacetic acid gave 2,2′-bipyridine-1-oxide, which was reacted with trimethylsilyl cyanide and benzoyl chloride to give the bipyridyl–carbonitrile compound. Condensation of the bipyridyl–carbonitrile with various amino alcohols in the presence of Zn(OTf)2 produced the bipyridiyl–oxazoline ligands 4a–4c in moderate yields. The neutral bipyridiyl–oxazoline cobalt(II) dichloride complexes 5a–5c were formed in high yield by the addition of the corresponding ligand to anhydrous cobalt dichloride in anhydrous tetrahydrofuran (THF). Similarly, terpyridine complex 5d was synthesised in the same manner from commercially available terpyridine. These cobalt complexes showed paramagnetically broadened and shifted resonances in the 1H NMR spectra (see ESI†).
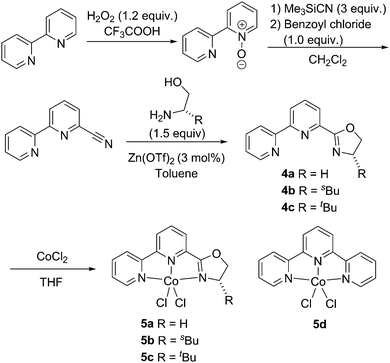 | ||
| Scheme 2 Synthesis of bipyridiyl–oxazoline cobalt complexes 5a–5c and terpyridine cobalt complex 5d. | ||
Initial studies focused on the hydroboration of styrene with pinacolborane (1.1 equiv.) using a range of cobalt pre-catalysts (1 mol%) and NaOtBu (2 mol%) as the in situ activator in tetrahydrofuran at room temperature (Table 1). Hydroboration of styrene using HBPOCoCl25a and sBuBPOCoCl25b gave the branched hydroboration product in good yield and with 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 and 82
30 and 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 branched
18 branched![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) linear regioselectivity, respectively (Table 1, entries 1 and 2). When the more bulky tBuBPOCoCl25c was used, hydroboration of styrene proceeded in excellent yield and with the highest selectivity for the Markovnikov regioisomer (97
linear regioselectivity, respectively (Table 1, entries 1 and 2). When the more bulky tBuBPOCoCl25c was used, hydroboration of styrene proceeded in excellent yield and with the highest selectivity for the Markovnikov regioisomer (97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) (Table 1, entry 3). Terpyridine cobalt complex 5d gave a moderate yield and good Markovnikov regioselectivity (91
3) (Table 1, entry 3). Terpyridine cobalt complex 5d gave a moderate yield and good Markovnikov regioselectivity (91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 1, entry 4). When the hydroboration of styrene, using tBuBPOCoCl25c, was performed in the absence of solvent, good Markovnikov selectivity (91
1) (Table 1, entry 4). When the hydroboration of styrene, using tBuBPOCoCl25c, was performed in the absence of solvent, good Markovnikov selectivity (91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 b/l) was also achieved, but with decreased yield (Table 1, entry 5). Hydroboration of styrene proceeded well in ethyl acetate and toluene, albeit in reduced yield and regioselectivity compared to that in THF (Table 1, entries 6 and 7). Catalyst activation in situ with EtMgBr and NaBHEt3 gave both lower yields and regioselectivities compared to the activation using NaOtBu (Table 1, entries 8 and 9). A control reaction using non-ligated anhydrous CoCl2 under the optimised reaction conditions, showed no catalytic activity (Table 1, entry 10). Similarly, the reaction did not proceed in the absence of cobalt pre-catalyst or without added NaOtBu (Table 1, entries 11 and 12).
1 b/l) was also achieved, but with decreased yield (Table 1, entry 5). Hydroboration of styrene proceeded well in ethyl acetate and toluene, albeit in reduced yield and regioselectivity compared to that in THF (Table 1, entries 6 and 7). Catalyst activation in situ with EtMgBr and NaBHEt3 gave both lower yields and regioselectivities compared to the activation using NaOtBu (Table 1, entries 8 and 9). A control reaction using non-ligated anhydrous CoCl2 under the optimised reaction conditions, showed no catalytic activity (Table 1, entry 10). Similarly, the reaction did not proceed in the absence of cobalt pre-catalyst or without added NaOtBu (Table 1, entries 11 and 12).
| Entry | [Co] (mol%) | Activator (mol%) | Solvent | Yieldb (%) | Branched![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) linear linear |
|---|---|---|---|---|---|
| a Standard reaction conditions: styrene (0.5 mmol), HBpin (1.1 equiv.), [Co] (1.0 mol%) and NaOtBu (2.0 mol%) in THF (3 ml) at 25 °C. b Yields determined by 1H NMR using 1,3,5-trimethoxybenzene as the internal standard. c 2% hydrogenation product observed. d 5% hydrogenation product observed. e 30% hydrogenation product observed. | |||||
| 1 | 5a(1) | NaOtBu(2) | THF | 82 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
| 2 | 5b(1) | NaOtBu(2) | THF | 87 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
| 3 | 5c(1) | NaOtBu(2) | THF | >95 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| 4 | 5d(1) | NaOtBu(2) | THF | 69 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
| 5 | 5c(1) | NaOtBu(2) | Neat | 59 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
| 6 | 5c(1) | NaOtBu(2) | EtOAc | 77c | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 7 | 5c(1) | NaOtBu(2) | Toluene | >95 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 8 | 5c(1) | EtMgBr(2) | THF | 85 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 9 | 5c(1) | NaBHEt3(2) | THF | 63d | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 10e | CoCl2(1) | NaOtBu(2) | THF | — | — |
| 11 | — | NaOtBu(2) | THF | — | — |
| 12 | 5a(1) | — | THF | 3 | n.d. |
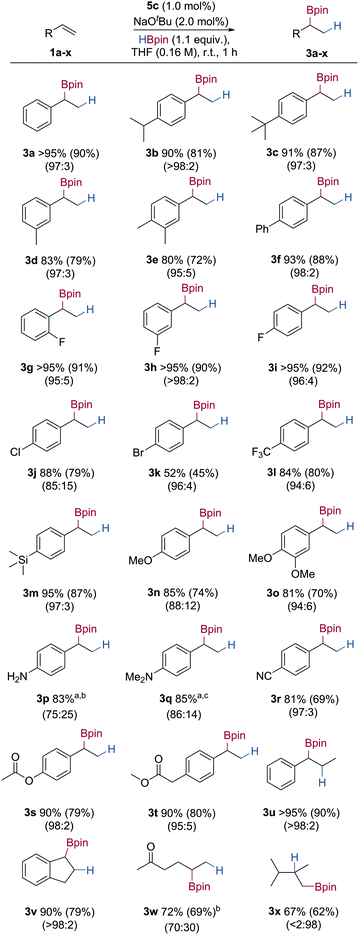
Using the optimised reaction conditions, the substrate scope of the Markovnikov selective hydroboration was explored using a range of electronically and sterically differentiated styrene derivatives (Table 2). Hydroboration of styrene gave the secondary boronic ester in excellent isolated yield, and a 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 branched
3 branched![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) linear selectivity (3a, 90%). Styrene derivatives bearing electron-donating groups such as iso-propyl, tert-butyl and methyl underwent successful hydroboration in excellent yield and regioselectivity (3b–3e, 72–87%). 4-Phenylstyrene gave the secondary boronic ester in good yield and regioselectivity (3f, 88%). Styrene derivatives bearing electron-withdrawing substituents including fluoro-, chloro-, bromo- and trifluoromethyl- also underwent successful hydroboration, giving the secondary boronic esters in moderate to excellent yields, with no cleavage of aryl–halide bond observed (3g–3l, 45–92%).19,20 Styrene derivatives bearing trialkylsilyl- and ether substituents also underwent successful hydroboration in good yields and selectivities to give the branched boronic esters (3m–3o, 70–87%). Hydroboration of primary and tertiary amine substituted substrates both gave the secondary boronic ester in good regioselectivity and yield (3p–3q, 83–85%). Styrene derivatives containing more than one unsaturated group, such as ester and nitrile substituents, were chemoselectively hydroborated at the alkene in excellent yield and regioselectivity, with no observed C–O or C–N bond cleavage, or reduction (3r–3t, 69–80%).21 Styrene derivatives bearing substituents at the β-position, such as trans-β-methylstyrene and indene were also reactive under the developed conditions, giving the product boronic esters with complete control of regioselectivity and in excellent yield (3u–3v, 79–90%). For alkyl alkenes, ketone containing alkene 1w successfully gave the branched boronic ester 3w in good yield with moderate regioselectivity and without ketone reduction. Hydroboration of 2,3-dimethyl-1-butene 1x gave the linear boronic ester 3x in good yield. Presumably, increased steric bulk of the 1,1-disubstituted alkene led to the anti-Markovnikov regioisomer. Oxidation of the secondary boronic ester products to the corresponding alcohols, and analysis by chiral HPLC, showed no enantioselectivity, indicating no stereoinduction from the ligand.
linear selectivity (3a, 90%). Styrene derivatives bearing electron-donating groups such as iso-propyl, tert-butyl and methyl underwent successful hydroboration in excellent yield and regioselectivity (3b–3e, 72–87%). 4-Phenylstyrene gave the secondary boronic ester in good yield and regioselectivity (3f, 88%). Styrene derivatives bearing electron-withdrawing substituents including fluoro-, chloro-, bromo- and trifluoromethyl- also underwent successful hydroboration, giving the secondary boronic esters in moderate to excellent yields, with no cleavage of aryl–halide bond observed (3g–3l, 45–92%).19,20 Styrene derivatives bearing trialkylsilyl- and ether substituents also underwent successful hydroboration in good yields and selectivities to give the branched boronic esters (3m–3o, 70–87%). Hydroboration of primary and tertiary amine substituted substrates both gave the secondary boronic ester in good regioselectivity and yield (3p–3q, 83–85%). Styrene derivatives containing more than one unsaturated group, such as ester and nitrile substituents, were chemoselectively hydroborated at the alkene in excellent yield and regioselectivity, with no observed C–O or C–N bond cleavage, or reduction (3r–3t, 69–80%).21 Styrene derivatives bearing substituents at the β-position, such as trans-β-methylstyrene and indene were also reactive under the developed conditions, giving the product boronic esters with complete control of regioselectivity and in excellent yield (3u–3v, 79–90%). For alkyl alkenes, ketone containing alkene 1w successfully gave the branched boronic ester 3w in good yield with moderate regioselectivity and without ketone reduction. Hydroboration of 2,3-dimethyl-1-butene 1x gave the linear boronic ester 3x in good yield. Presumably, increased steric bulk of the 1,1-disubstituted alkene led to the anti-Markovnikov regioisomer. Oxidation of the secondary boronic ester products to the corresponding alcohols, and analysis by chiral HPLC, showed no enantioselectivity, indicating no stereoinduction from the ligand.
Conditions: alkene (0.5 mmol), HBpin (1.1 equiv.), 5c (1.0 mol%) and NaOtBu (2.0 mol%) in THF (3 mL) at 25 °C, 1 h. Yield was determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as an internal standard. Isolated yields in parentheses. Branched![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) linear ratios determined from integrals of product peaks in 1H NMR of crude reaction mixtures.a Product decomposed on SiO2.b 10% alkene hydrogenation product observed in the crude reaction mixture.c 8% alkene hydrogenation product observed in the crude reaction mixture. linear ratios determined from integrals of product peaks in 1H NMR of crude reaction mixtures.a Product decomposed on SiO2.b 10% alkene hydrogenation product observed in the crude reaction mixture.c 8% alkene hydrogenation product observed in the crude reaction mixture. |
|---|
|
In order to gain insight into the mechanism of the Markovnikov selective hydroboration of vinylarenes with HBpin, deuterium labelling experiments were performed. When the hydroboration of styrene was carried out in d8-THF, only the fully protio-boronic ester 3a was obtained, indicating that both H and Bpin originate from pinacolborane (Scheme 3A). When the hydroboration of d8-styrene with HBpin was performed in THF, the mono-protio-boronic ester d8-3a was formed exclusively with ‘H’ at the terminal methyl group (Scheme 3B). When DBpin (96% D-content) was used in the hydroboration of styrene, a mixture of mono-deuterated boronic ester d1-3a and fully protio-boronic ester 3a was formed, in a ratio of 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 D/H (Scheme 3C). The presence of (fully) protio-3a presumably arises from either β-hydride elimination of an intermediate organo-cobalt species,22 to generate a cobalt hydride that serves to add ‘H’ to styrene 1a, or H/D exchange with extraneous water, either present in the reaction medium or on work-up.
12 D/H (Scheme 3C). The presence of (fully) protio-3a presumably arises from either β-hydride elimination of an intermediate organo-cobalt species,22 to generate a cobalt hydride that serves to add ‘H’ to styrene 1a, or H/D exchange with extraneous water, either present in the reaction medium or on work-up.
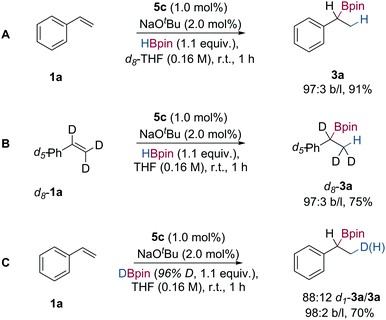 | ||
| Scheme 3 Deuterium labelling studies of Markovnikov selective styrene hydroboration; isolated yields following flash column chromatography. | ||
In conclusion, we have developed a Markovnikov selective cobalt-catalysed hydroboration of alkenes with pinacolborane at ambient temperature, using a bench-stable cobalt pre-catalyst bearing a bipyridiyl–oxazoline ligand. This strategy has been applied to a variety of electronically and sterically differentiated styrene derivatives, bearing a range of functional groups, to give the secondary boronic esters in both high yield and regioselectivity.
S. P. T. acknowledges the University of Edinburgh for a Chancellor's Fellowship and the Royal Society for a University Research Fellowship and a Research Grant. J. P. acknowledges the University of Edinburgh and China Scholarship Council for a joint scholarship. J. H. D. and S. P. T. acknowledge GlaxoSmithKline and the EPSRC (EP/M506515/1) for a PhD studentship.
Notes and references
- (a) H. C. Brown, Organic Synthesis via Boranes, Wiley, New York, 1975 Search PubMed; (b) H. C. Brown and B. Singaram, Pure Appl. Chem., 1987, 59, 879 CAS; (c) Science of Synthesis: Vol. 6, Boron Compounds, ed. D. E. Kaufmann and D. S. Matteson, Georg Thieme Verlag, Stuttgart-New York, 2004 Search PubMed; (d) Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine, ed. D. G. Hall, Wiley-VCH, Weinheim, 2005 Search PubMed.
- (a) K. Burgess and M. J. Ohlmeyer, Chem. Rev., 1991, 91, 1179 CrossRef CAS; (b) I. Beletskaya and A. Pelter, Tetrahedron, 1997, 53, 4957 CrossRef CAS; (c) C. M. Crudden and D. Edwards, Eur. J. Org. Chem., 2003, 4695 CrossRef CAS.
- M. D. Greenhalgh, A. S. Jones and S. P. Thomas, ChemCatChem, 2014, 6, 1520 CrossRef CAS.
- I. Bauer and H.-J. Knölker, Chem. Rev., 2015, 115, 3170 CrossRef CAS PubMed.
- L. Zhang, D. Peng, X. Leng and Z. Huang, Angew. Chem., Int. Ed., 2013, 52, 3676 CrossRef CAS PubMed.
- J. V. Obligacion and P. J. Chirik, J. Am. Chem. Soc., 2013, 135, 19107 CrossRef CAS PubMed.
- W. N. Palmer, T. Diao, I. Pappas and P. J. Chirik, ACS Catal., 2015, 5, 622 CrossRef CAS.
- S. W. Reilly, C. E. Webster, T. K. Hollis and H. U. Valle, Dalton Trans., 2016, 45, 2823 RSC.
- M. L. Scheuermann, E. J. Johnson and P. J. Chirik, Org. Lett., 2015, 17, 2716 CrossRef CAS PubMed.
- M. D. Greenhalgh and S. P. Thomas, Chem. Commun., 2013, 49, 11230 RSC.
- J. Chen, T. Xi and Z. Lu, Org. Lett., 2014, 16, 6452 CrossRef CAS PubMed.
- A. J. MacNair, C. R. P. Millet, G. S. Nichol, A. Ironmonger and S. P. Thomas, ACS Catal., 2016, 6, 7217 CrossRef CAS.
- M. Espinal-Viguri, C. R. Woof and R. L. Webster, Chem. – Eur. J., 2016, 22, 11605 CrossRef CAS PubMed.
- J. Chen, T. Xi, X. Ren, B. Cheng, J. Guo and Z. Lu, Org. Chem. Front., 2014, 1, 1306 RSC.
- T. Xi and Z. Lu, ACS Catal., 2017, 7, 1181 CrossRef CAS.
- E. E. Touney, R. Van Hoveln, C. T. Buttke, M. D. Freidberg, I. A. Guzei and J. M. Schomaker, Organometallics, 2016, 35, 3436 CrossRef CAS.
- S. Krautwald, M. J. Bezdek and P. J. Chirik, J. Am. Chem. Soc., 2017, 139, 3868 CrossRef CAS PubMed.
- J. H. Docherty, J. Peng, A. P. Dominey and S. P. Thomas, Nat. Chem. DOI:10.1038/nchem.2697.
- T. Hatakeyama, Y. Kondo, Y. Fujiwara, H. Takaya, S. Ito, E. Nakamura and N. Nakamura, Chem. Commun., 2009, 1216 RSC.
- W. M. Czaplik, S. Grupe, M. Mayer and A. Jacobi von Wangelin, Chem. Commun., 2010, 46, 6350 RSC.
- R. J. Trovitch, E. Lobkovsky, M. W. Bouwkamp and P. J. Chirik, Organometallics, 2008, 27, 6264 CrossRef CAS.
- It should be noted that we were unable to observe any products of H/D scrambling consistent with this scenario (e.g. dn-styrene) in the crude reaction mixtures, but this may be due to loss of these low boiling point products on work-up.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cc01085k |
| This journal is © The Royal Society of Chemistry 2017 |