Open Access Article
Open Access ArticleOne-pot conversion of biomass-derived xylose and furfural into levulinate esters via acid catalysis†
Xun
Hu
 ,
Shengjuan
Jiang
,
Liping
Wu
,
Shuai
Wang
and
Chun-Zhu
Li
*
,
Shengjuan
Jiang
,
Liping
Wu
,
Shuai
Wang
and
Chun-Zhu
Li
*
Fuels and Energy Technology Institute, Curtin University of Technology, GPO Box U1987, Perth, WA 6845, Australia. E-mail: chun-zhu.li@curtin.edu.au; Tel: +61 8 9266 1131
First published on 15th February 2017
Abstract
Direct conversion of biomass-derived xylose and furfural into levulinic acid, a platform molecule, via acid-catalysis has been accomplished for the first time in dimethoxymethane/methanol. Dimethoxymethane acted as an electrophile to transform furfural into 5-hydroxymethylfurfural (HMF). Methanol suppressed both the polymerisation of the sugars/furans and the Aldol condensation of levulinic acid/ester.
Levulinic acid is a building block chemical for the production of various value-added chemicals such as pharmaceuticals, plasticizers and various other additives.1 Commercially, levulinic acid is produced from the hydrolysis of biomass in the presence of a mineral acid with yields ranging from 10 to 30 wt%.2 The low yields of levulinic acid from biomass originate mainly from (1) the polymerisation of sugars during the conversion into levulinic acid3 and (2) the fact that only the C6 sugar substrates (glucose unit) in cellulose and hemicelluloses (mannose and galactose units) can be potentially converted into levulinic acid via acid catalysis.4 The C5 sugar substrates (xylose and arabinose units) under the same conditions are mainly converted into furfural,5 which is an intrinsic reason for the low yields of levulinic acid from biomass.
C5 sugars like xylose can be converted into levulinic acid,6 but via multiple steps, including the initial acid-catalyzed conversion to produce furfural in the liquid phase,7 the following partial hydrogenation of furfural to furfuryl alcohol in the gas8 or liquid phase9 and the subsequent acid-catalyzed conversion of furfuryl alcohol into levulinic acid/ester in the liquid phase.10 The above process involves both hydrogenation and acid-catalyzed reactions. It is preferable to avoid the hydrogenation step as it involves the use of hydrogenation catalysts, high pressure and hydrogen, making the process complicated and costly. A new strategy needs to be developed to achieve direct conversion of C5 sugars into levulinic acid under mild conditions.
The structural difference between HMF, the dehydration intermediate of C6 sugars,11 and furfural, the dehydration intermediate of C5 sugars,12 is the additional hydroxymethyl group in HMF. If one hydroxymethyl group could be added to the furan ring in furfural, furfural would then be able to be transformed into HMF, and subsequently into levulinic acid. The furan ring in furfural has aromaticity, and it is thus possible to introduce the hydroxymethyl group to furfural via electrophilic substitution.
In this study, we developed dimethoxymethane (DMM)/methanol as an effective reaction medium to transform xylose directly into levulinic acid/ester via only acid catalysis. The overall reaction pathways for the conversion of xylose into levulinic acid via acid catalysis are proposed in Scheme 1. The yields of levulinic acid/ester reached ca. 52% (150 °C, Amberlyst 70, in DMM/methanol), which was comparable to the yields of levulinic acid from glucose in water (ca. 50%, 180 °C, Amberlyst 70, in water).13 These are the new reaction routes for the direct conversion of xylose and furfural into levulinic acid under mild reaction conditions.
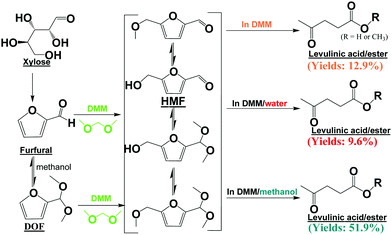 | ||
| Scheme 1 Conversion of xylose to levulinic acid/ester in DMM/co-solvent. All the products were detected using GC-MS. | ||
Entries 1–5 in Table 1 show the effects of reaction temperature on the formation of levulinic ester (LE) and levulinic acid (LA) from xylose in dimethoxymethane (DMM). The electrophilic substitution between DMM and furfural took place, transforming furfural into HMF or its derivatives (Fig. S1a in the ESI†). The concept of transforming furfural into HMF via electrophilic substitution in DMM was confirmed. However, the formation of LA or LE from xylose was not selective and the yields further decreased at higher reaction temperatures.
| Entry | Key parameters | Yields (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Reactants | Reaction medium | T (°C) | Catalyst(s) | t (min) | LE | LA | LE + LA | |
a Other reaction conditions: saccharide: 1.8 g; Amberlyst 70 (A70): 3.6 g; solvent: 40 mL; t = 120 min; p = autogenous vapour pressure.
b The volume ratio of the solvents. The volumetric ratio for the mixed solvents with no parenthesis was 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15.
c La(III) stands for lanthanum(III) trifluoromethanesulfonate. The distribution of other products is summarized in Table S1 in the ESI. 15.
c La(III) stands for lanthanum(III) trifluoromethanesulfonate. The distribution of other products is summarized in Table S1 in the ESI.
|
||||||||
| 1 | Xylose | DMM | 140 | A70 | 120 | 13.9 | 1.2 | 15.1 |
| 2 | Xylose | DMM | 150 | A70 | 120 | 12.2 | 0.7 | 12.9 |
| 3 | Xylose | DMM | 160 | A70 | 120 | 6.4 | 1.6 | 8.0 |
| 4 | Xylose | DMM | 170 | A70 | 120 | 1.6 | 1.4 | 3.0 |
| 5 | Xylose | DMM | 180 | A70 | 120 | 0.2 | 0.1 | 0.3 |
| 6 | Xylose | DMM/water | 150 | A70 | 120 | 5.0 | 4.6 | 9.6 |
| 7 | Xylose | DMM/DMSO | 150 | A70 | 120 | 0.2 | 0.4 | 0.6 |
| 8 | Xylose | DMM/THF | 150 | A70 | 120 | 14.5 | 1.6 | 16.1 |
| 9 | Xylose | DMM/diethyl ether | 150 | A70 | 120 | 13.6 | 0.9 | 14.5 |
| 10 | Xylose | DMM/toluene | 150 | A70 | 120 | 19.7 | 1.7 | 21.4 |
| 11 | Xylose | DMM/iso-propanol | 150 | A70 | 120 | 10.0 | 7.1 | 17.1 |
| 12 | Xylose | DMM/methyl formate | 150 | A70 | 120 | 18.0 | 1.4 | 19.4 |
| 13 | Xylose | DMM/ethanol | 150 | A70 | 120 | 22.6 | 2.1 | 24.7 |
| 14 | Xylose | DMM/methanol (25/15)b | 150 | A70 | 120 | 22.8 | 2.1 | 24.9 |
| 15 | Xylose | DMM/methanol (32.5/7.5) | 150 | A70 | 120 | 29.1 | 3.0 | 32.1 |
| 16 | Xylose | DMM/methanol (29/11) | 150 | A70 | 120 | 32.6 | 2.2 | 34.8 |
| 17 | Xylose | DMM/methanol (15/25) | 150 | A70 | 120 | 12.8 | 1.2 | 13.0 |
| 18 | Xylose | DMM (40/0) | 160 | A70 | 120 | 6.4 | 1.6 | 8.0 |
| 19 | Xylose | DMM/methanol (25/15) | 160 | A70 | 120 | 37.7 | 3.8 | 41.5 |
| 20 | Xylose | DMM/methanol | 150 | A70 | 360 | 41.6 | 10.3 | 51.9 |
| 21 | Xylose | DMM/methanol | 160 | La(III)c | 120 | 3.1 | 0.3 | 3.4 |
| 22 | Xylose | DMM/methanol | 160 | La(III) + A70 | 120 | 33.2 | 8.4 | 41.6 |
| 23 | Xylose | Acetaldehyde | 160 | A70 | 120 | 0 | 0 | 0 |
| 24 | Xylose | DEM/water | 160 | A70 | 120 | 4.4 | 2.6 | 7.0 |
| 25 | Xylose | DEM/methanol | 160 | A70 | 120 | 27.4 | 4.0 | 31.4 |
| 26 | Xylose | DEM/ethanol | 160 | A70 | 120 | 21.8 | 2.5 | 24.3 |
| 27 | Glucose | DMM/methanol | 160 | A70 | 120 | 52.5 | 11.0 | 63.5 |
| 28 | Glucose + xylose | DMM/methanol | 160 | A70 | 120 | 40.7 | 8.4 | 49.1 |
| 29 | Furfural | DMM/methanol | 160 | A70 | 120 | 43.3 | 3.5 | 46.8 |
| 30 | Furfural | DMM | 160 | A70 | 120 | 27.3 | 2.8 | 30.0 |
| 31 | Furfural | DMM/water | 160 | A70 | 120 | 16.1 | 10.4 | 26.5 |
| 32 | HMF | DMM/methanol | 160 | A70 | 120 | 61.7 | 11.9 | 73.6 |
Furfural is highly reactive towards polymerization.14 Soluble polymers in the products were detected and their abundance increased with the reaction temperatures (Fig. S2, ESI†). Abundant ketone functionalities in the insoluble polymer were detected using FT-IR, especially above 150 °C (Fig. S3, ESI†). The polymerization of furfural was suspected to be the main reason for the low yields of levulinic acid/ester. DMM as the solvent/reactant alone was not able to prevent the polymerization. Co-solvents were thus employed herein to tackle the polymerizations of xylose/furfural in DMM.
Entries 6–14 in Table 1 show the effects of co-solvents on the yields of levulinic acid/ester. It was found that toluene also reacted with DMM to form aromatics (Scheme S1, ESI†), which was similar to the formation of HMF from furfural in terms of mechanism. This is because both the furan and benzene rings have aromaticity, and they could react with DMM via electrophilic substitution reactions. The reaction pathways for the transformation of furfural into the derivative of HMF are proposed in Scheme S2 (ESI†). Among the co-solvents investigated, methanol or ethanol could remarkably promote the production of levulinic acid/ester (entries 13 and 14). The positive effects of alcohols on levulinic acid/ester formation were further investigated.
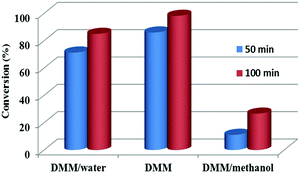
Entries 14–19 in Table 1 show the effects of methanol content in the reaction medium on levulinic acid/ester production. With the addition of a small amount of methanol to the reaction medium, the yields of levulinic acid/ester were more than doubled (entry 15 versus entry 2). Methanol could react with furfural, forming 2-(dimethoxymethyl)furan (DOF, the acetal of furfural) (Scheme 1). The formation of DOF destroyed the π-conjugation between the furan ring and the carbonyl group, which possibly made the furan ring more susceptible to the attack by the electrophile, facilitating the subsequent electrophilic substitution reaction. More importantly, methanol significantly suppressed the polymerization reactions. As evidenced in Fig. 1a, the abundance of the soluble polymers decreased remarkably with methanol as the co-solvent.
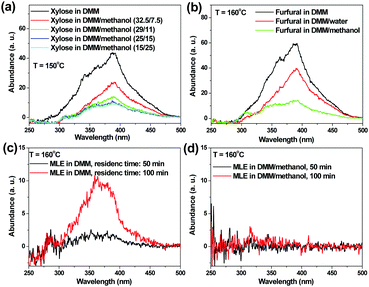 | ||
| Fig. 1 Constant energy (−2800 cm−1) synchronous spectra of the soluble polymers formed from the acid-catalyzed conversion of xylose, furfural and methyl levulinate (MLE) in DMM or DMM/methanol. | ||
Entries 20–28 in Table 1 show the conversion of xylose/glucose versus reaction time, catalysts and electrophiles. The formation of levulinic acid/ester was favored by a longer residence time (entry 20). The Lewis acid catalyst was not active for the conversion of xylose to levulinic acid/ester (entry 21), but was active for the polymerization reactions (Fig. S4, ESI†). A combination of the Lewis acid with A70 (Brønsted acid) did not improve the performance (entry 22 versus entry 19). Acetaldehyde as the electrophile could not trigger the transformation of furfural into HMF. In diethoxymethane (DEM)/water, the levulinate ester produced was switched to ethyl levulinate (entry 9), indicating that the ethoxy group from DEM was transferred into the product. Methanol or ethanol as the co-solvent of DEM promoted the formation of the levulinic esters.
When glucose was used as a reactant, levulinic acid/ester yields reached ca. 63.5% (entry 27), which was higher than that in water (ca. 50%).13 For mixed glucose/xylose, half of them were converted to levulinic acid/ester (entry 28). In this process, neither hydrogen nor a hydrogenation catalyst was required. Using only an acid catalyst and DMM/methanol, the simultaneous conversion of both the C5 and C6 sugars into levulinic acid/ester was achieved. The conversion of the biomass-derived furans into levulinic acid/ester was also investigated in DMM/methanol.
Entries 29–32 in Table 1 show the formation of levulinic acid/ester from furfural and HMF. With DMM/methanol as the reaction medium, the polymerization reactions could be effectively suppressed (Fig. 1b). However, the yields of levulinic acid/ester from furfural could not reach 50% (entry 29), which was similar to that from xylose (entry 19).
Clearly, whether xylose or furfural was used as the starting reactant made no marked difference in terms of the yields of levulinic acid/ester (entries 19 and 29), which was also confirmed by the conversion of xylose or furfural versus the reaction time (Fig. S5–S7, ESI†). Thus, the polymerization during the conversion of xylose to furfural was not the main reason for the loss of levulinic acid/ester production. Other undesirable reactions must be responsible. The soluble polymers formed from furfural or HMF in DMM/methanol were very different from those in water (Fig. S8, ESI†), indicating the involvement of polymerization other than that from xylose or furfural.
Levulinic acid and methyl levulinate have a ketonic group and active α-H, making them very active in polymerization reactions. It was found that the formation of levulinic acid/ester from xylose (Fig. S5, ESI†) or furfural (Fig. S6, ESI†) was always accompanied by the formation of methyl 3-methylene-4-oxopentanoate (MMO) (Fig. S1, ESI†) and polymers (Fig. S7 and S8, ESI†), especially at elevated reaction temperatures where the yields of levulinic acid/ester were low. MMO was identified by interpretation of its mass spectrum (Schemes S3 and S4, ESI†), which was produced via the Aldol condensation between methyl levulinate and DMM. Other compounds formed via similar routes were also identified (Scheme 2).
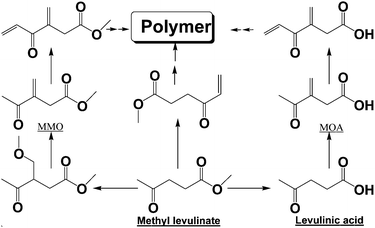 | ||
| Scheme 2 Condensation of methyl levulinate in DMM. All the compounds except the polymer were detected using GC-MS. | ||
The formation of MMO and other condensation products consumed the methyl levulinate produced and led to the polymer formation (Fig. S7, ESI†), which diminished the production of levulinic acid/ester from xylose or furfural. The abundance of MMO in DMM was thrice as high as that in DMM/methanol. Evidently, the presence of methanol suppressed the Aldol condensation of levulinic acid/ester and preserved them in the reaction medium. This was further verified by the experiments with methyl levulinate as the starting reactant.
With methanol as the co-solvent, although MMO was still formed (Table S2, ESI†), the conversion of methyl levulinate was much smaller (Fig. 2). Evidently, the polymerization of methyl levulinate was suppressed in the presence of methanol. Aldol condensation was the main route for the polymerization of methyl levulinate in DMM, leading to the formation of the polymers with conjugated π-structures and extended size (Fig. 1c). Herein we demonstrated that methanol could suppress the Aldol condensation reaction, suppressing the polymer formation (Fig. 1d) and preserving levulinic acid/ester in the reaction medium.
Other reaction parameters in the conversion of xylose/furfural in DMM/methanol were also investigated. The mass transfer limitation was insignificant at stirring rates above 300 rpm (Fig. S9, ESI†). A70 has a limited capacity to catalyse the conversion of xylose with high loading (Fig. S10, ESI†), due to deactivation induced by the polymer formation (Fig. S11 and S12, ESI†). The recycle tests with xylose (Fig. S13, ESI†) or furfural (Fig. S14, ESI†) as the starting reactant confirmed the deactivation of A70. Both soluble (Fig. S15 and S16, ESI†) and insoluble polymers (Fig. S17, ESI†) were formed, which significantly reduced the acid density of A70 (Fig. S18, ESI†). The functionalities of the soluble polymer include aromatic ring structures, α–β unsaturated carbonyls and carbon double bonds (Fig. S19, ESI†). The insoluble polymer has similar functionalities (Fig. S17, ESI†) and thermal stability to A70 (Fig. S20, ESI†).
To summarize, a new route for the one-pot conversion of biomass-derived C5 sugars and furans into levulinic acid/ester has been demonstrated. The method is very simple. No hydrogen or hydrogenation catalyst is needed. Only an acid catalyst with DMM/methanol as the reactant/solvent is required to achieve the direct conversion of the C5 sugars to levulinic acid/ester. In this method, DMM is the electrophile to transform furfural into HMF via electrophilic substitution reactions. Methanol as the co-solvent/reactant played multiple critical roles: (1) it promoted the electrophilic substitution of furfural to produce HMF or the derivatives of HMF; (2) it suppressed the polymerization reactions of the sugars in the acidic reaction medium; and (3) it suppressed the Aldol condensation of levulinic acid/ester with DMM. Using the developed method, the cellulose-derived C6 sugars, the hemicelluloses-derived C5 sugars and the furans (furfural, HMF) all can be converted to the same products, levulinic acid/ester, via the same acid catalysis process. This drastically enhances the efficiency for the production of levulinic acid/ester from biomass, the platform molecules for chemical diversity and biofuel production.
This project was supported by the Commonwealth of Australia under the Australia–China Science and Research Fund as well as Curtin University of Technology through the Curtin Research Fellowship Scheme. This project received funding from ARENA as part of ARENA's Emerging Renewables Program.
Notes and references
- J. P. M. Sanders, J. H. Clark, G. J. Harmsen, H. J. Heeres, J. J. Heijnen, S. R. A. Kersten, W. P. M. Swaaij and J. A. Moulijn, Chem. Eng. Process., 2012, 51, 117 CrossRef CAS; D. M. Alonso, J. Q. Bond and J. A. Dumesic, Green Chem., 2010, 12, 1493 RSC; G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044 CrossRef PubMed.
- M. R. Galletti, C. Antonetti, V. D. Luise, D. Licursi and N. Nassi, BioResources, 2012, 7, 1824 Search PubMed.
- X. Hu and C.-Z. Li, Green Chem., 2011, 13, 1676 RSC.
- C. Chatterjee, F. Pong and A. Sen, Green Chem., 2015, 17, 40 RSC; J. Song, L. Wu, B. Zhou, H. Zhou, H. Fan, Y. Yang, Q. Meng and B. Han, Green Chem., 2015, 17, 1626 RSC.
- G. Mazzotta, D. Gupta, B. Saha, A. K. Patra, A. Bhaumik and M. M. Abu-Omar, ChemSusChem, 2014, 7, 2342 CrossRef PubMed; S. Dias, M. Pillinger and A. A. Valente, Appl. Catal., A, 2005, 285, 126 CrossRef.
- J.-P. Lange, E. Heide, J. Buijtenen and R. Price, ChemSusChem, 2012, 5, 150 CrossRef CAS PubMed.
- R. Weingarten, J. Cho, W. C. Conner, Jr. and G. W. Huber, Green Chem., 2010, 12, 1423 RSC; C. García-Sancho, I. Sádaba, R. Moreno-Tost, J. Mérida-Robles, J. Santamaría-González, M. López-Granados and P. Maireles-Torres, ChemSusChem, 2013, 6, 635 CrossRef CAS PubMed.
- K. Fulajtarova, T. Sotak, M. Hronec, I. Vavra, E. Dobrocka and M. Omastova, Appl. Catal., A, 2015, 502, 78 CrossRef CAS.
- M. M. Villaverde, N. M. Bertero, T. F. Garetto and A. J. Marchi, Catal. Today, 2013, 213, 87 CrossRef CAS.
- G. M. G. Maldonado, R. S. Assary, J. Dumesic and L. A. Curtiss, Energy Environ. Sci., 2012, 5, 6981 Search PubMed; J. P. Lange, W. D. Graaf and R. J. Haan, ChemSusChem, 2009, 2, 437 CrossRef CAS PubMed; X. Hu, Y. Song, L. Wu, M. Gholizadeh and C.-Z. Li, ACS Sustainable Chem. Eng., 2013, 1, 1593 CrossRef.
- T. Deng, X. Cui, Y. Qi, Y. Wang, X. Hou and Y. Zhu, Chem. Commun., 2012, 48, 5494 RSC; G. R. Akien, L. Qi and I. T. Horváth, Chem. Commun., 2012, 48, 5850 RSC; W. Deng, M. Liu, Q. Zhang, X. Tan and Y. Wang, Chem. Commun., 2010, 46, 2668 RSC.
- X. Tong, Z. Liu, L. Yu and Y. Li, Chem. Commun., 2015, 51, 3674 RSC; M. A. Harmer, A. Fan, A. Liauw and R. K. Kumar, Chem. Commun., 2009, 6610 RSC; S. Dias, M. Pillinger and A. A. Valente, Appl. Catal., A, 2005, 285, 126 CrossRef CAS.
- X. Hu, S. Wang, L. Wu, D. Dong, M. M. Hasan and C.-Z. Li, Fuel Process. Technol., 2014, 126, 315 CrossRef CAS.
- I. Zandvoort, Y. Wang, C. B. Rasrendra, E. R. H. Eck, P. C. A. Bruijnincx, H. J. Heeres and B. M. Weckhuysen, ChemSusChem, 2013, 6, 1745 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cc01078h |
| This journal is © The Royal Society of Chemistry 2017 |