Open Access Article
Open Access ArticleBithienopyrroledione vs. thienopyrroledione based copolymers: dramatic increase of power conversion efficiency in bulk heterojunction solar cells†
Xiaolan
Qiao‡
a,
Weichao
Chen‡
b,
Qinghe
Wu
a,
Shiqian
Zhang
c,
Hongzhuo
Wu
a,
Zhiqiang
Liu
 c,
Renqiang
Yang
c,
Renqiang
Yang
 *b and
Hongxiang
Li
*b and
Hongxiang
Li
 *a
*a
aKey Laboratory of Synthetic and Self-assembly Chemistry for Organic Functional Molecules, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 200032, China. E-mail: lhx@mail.sioc.ac.cn
bCAS Key Laboratory of Bio-based Materials, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, Qingdao, 266101, China. E-mail: yangrq@qibebt.ac.cn
cState Key Laboratory of Crystal Materials, Shandong University, Jinan 250100, China
First published on 6th March 2017
Abstract
Bithienopyrroledione (bi-TPD) based polymers P1 and P2 are designed and synthesized. Photovoltaic devices based on P1:PC71BM and P2:PC71BM blend films show power conversion efficiencies (PCEs) of 8.22% and 9.08%, respectively, whereas devices based on their thienopyrroledione (TPD)-based analogue P3 and PC71BM blend films display a PCE of 5.10%.
Polymer solar cells (PSCs) have been intensively investigated in recent years due to their features of low cost, flexibility, and light weight.1–5 The development in active layer materials, interfacial materials, and device processing technologies has improved the power conversion efficiencies (PCEs) of PSCs greater than 10%.6–12 In view of the active layer materials, donor–acceptor (D–A) type polymers have attracted great attention due to their advantages in tuning the absorption spectra, HOMO/LUMO energy levels, intermolecular interactions and molecular orientations, and hence numerous D–A type semiconductor polymers have been synthesized and used as donors in bulk heterojunction (BHJ) structure solar cells.13,14 To date, D–A polymers with PCEs over 8% have rarely been reported and most of them employ benzo[1,2,5]thiadiazole and fluorinated thieno[3,4-b]thiophene derivatives as electron deficient units.15–24 Thus, the exploration of new electron-deficient units (acceptor units) is urgently required for the development of high performance D–A polymers and the study of the relationships between polymer chemical structures and properties.
Thieno[3,4]pyrrole-4,6-dione (TPD) based polymers are widely used as electron donors in bulk heterojunction solar cells and a record PCE of 8.5% has been reported for TPD–benzodithiophene copolymerized polymers by using a classical device configuration.25–28 As a derivative of TPD, bithienopyrroledione (1,1′-bithieno[3,4-c]pyrrole-4,4′,6,6′-tetraone, bi-TPD) has a good planar structure and stronger electron-withdrawing ability than TPD,29–31 making it a good candidate as an acceptor unit for D–A polymers. However, bi-TPD based D–A polymers have rarely been investigated in PSCs. This might be partially because of the commonly accepted view that the large internal dipole moment along the polymer chain facilitates charge separation in PSCs and thus results in high PCE, which were examined by Yu's and Tsige's groups on polymers PTB7 and PBB3.32,33 In this study, we designed and synthesized two bi-TPD based polymers P1 and P2 (Scheme 1) and investigated their application as donor materials in PSCs. The P1 and P2 based cells exhibit PCEs of 8.22% and 9.08% with a traditional device configuration of ITO/PEDOT:PSS/P1 (P2):PC71BM/Ca/Al, much higher than that of the cell of TPD based analogue P3 (PCE: 5.10%) and the highest PCE value reported for bi-TPD contained polymers. These results demonstrate that bi-TPD based polymers have great potential for application in PSCs.
P1 and P2 were synthesized through conventional Stille coupling of a stannyl reagent of thieno[3,2-b]thiophene with the corresponding 5,5′-bis[5-bromo-3-akylthiophen]-1,1′-bithieno[3,4-c]pyrrole-4,4′,6,6′-tetraone. For comparison, P334 and P4, the mono-TPD based analogues of P1 and P2, were also synthesized using the Stille coupling reaction (Scheme S1 in the ESI†). Their number-average molecular weights (Mn) were measured using high temperature (150 °C) gel-permeation chromatography (GPC) in 1,2,4-trichlorobenzene using polystyrene as the standard. The Mn/PDI (polydispersity index) values were 82.9 kDa/2.88 for P1, 53.3 kDa/2.29 for P2, 154 kDa/4.99 for P3 and 7.1 kDa/2.83 for P4. The low molecular weight of P4 was due to its poor solubility, and solar cell devices based on P4 were not fabricated and investigated because of its lower Mn.
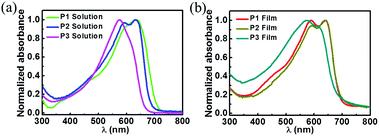
The UV-vis absorption spectra of copolymers P1–P3 were measured by a UV-vis spectrophotometer in o-dichlorobenzene (o-DCB) solution and in thin films spin-coated from o-DCB solution (Fig. 1). All polymers showed 0–0/0–1 peaks in solution and in thin films. For P1, the 0–1 peaks in solution and films were located at 588 nm, and the 0–0 peak was at 634 nm in solution and at 640 nm in films. For P2, similar to P1, the 0–1 peaks were at 592 nm in solution and films, and the 0–0 peak was shifted from 632 nm in solution to 640 nm in films. The 0–1 and 0–0 absorptions of P3 were at 575 and 614 nm in both solution and thin films. The optical band gaps estimated from thin film absorptions were 1.80, 1.80, and 1.84 eV for P1, P2, and P3, respectively. The electrochemical properties of the P1 and P2 thin films were investigated through cyclic voltammetry (CV) (Fig. S1, ESI†). P1 and P2 displayed a quasi-reversible oxidation process with redox potentials located at 1.24 and 1.22 V vs. SCE, respectively, and the HOMO energy level was −5.61 eV for P1 and −5.59 eV for P2. The LUMO energy levels calculated from the HOMO levels and optical band gaps were −3.81 eV for P1 and 3.79 eV for P2. The HOMO/LUMO energy levels of P1 and P2 were much lower than that of P3 (HOMO/LUMO: −5.07/−3.23 eV).34 The energy levels of the P1–P3 and PC71BM (LUMO: −4.30 eV; HOMO: −6.00 eV) exhibited a suitable cascade stage for exciton dissociation, suggesting their potential application in polymer:PC71BM blend solar cells.
The photovoltaic properties of polymers P1–P3 were investigated using the conventional device structure of ITO/PEDOT:PSS/polymer:PC71BM/Ca/Al. The current density–voltage (J–V) characteristics of the optimized devices are illustrated in Fig. 2a. The performance of the device was first optimized by using different polymer:PC71BM weight ratios (Table S1, ESI†). The optimal weight ratio for bi-TPD based polymers P1 and P2 to PC71BM was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and that for P3 to PC71BM was 1
2, and that for P3 to PC71BM was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5. Then, the effect of the 1,8-diiodooctane (DIO) additive on the device performance was investigated (Fig. S2–S4 and Table S2, ESI†). The experimental results show that (Table 1) the cells of P1:PC71BM with 1% DIO exhibited an optimal PCE of 8.22% with a Voc of 0.91 V, a Jsc of 13.45 mA cm−2, and a FF of 67.18%. For P2:PC71BM based devices, a PCE up to 9.08% with a Voc of 0.89 V, a Jsc of 14.15 mA cm−2, and a FF of 72.13% was observed upon addition of 0.25% DIO. For P3:PC71BM based devices, the optimal PCE was 5.10% with a Voc of 0.69 V, a Jsc of 11.70 mA cm−2, and a FF of 63.15%. This PCE value is comparable to the reported TPD and 2,5-di(thiophen-2-yl)thieno[3,2-b]thiophene (DT-TT) based polymers.35 Apparently, the Voc of P1 and P2 based solar cells was significantly larger than that of P3 based devices. This is consistent well with the deeper HOMO energy levels of the bi-TPD based polymers. The PCEs of P1 and P2 based devices may be further improved through device engineering similarly to that of PTB7-Th:PC71BM based solar cells.
1.5. Then, the effect of the 1,8-diiodooctane (DIO) additive on the device performance was investigated (Fig. S2–S4 and Table S2, ESI†). The experimental results show that (Table 1) the cells of P1:PC71BM with 1% DIO exhibited an optimal PCE of 8.22% with a Voc of 0.91 V, a Jsc of 13.45 mA cm−2, and a FF of 67.18%. For P2:PC71BM based devices, a PCE up to 9.08% with a Voc of 0.89 V, a Jsc of 14.15 mA cm−2, and a FF of 72.13% was observed upon addition of 0.25% DIO. For P3:PC71BM based devices, the optimal PCE was 5.10% with a Voc of 0.69 V, a Jsc of 11.70 mA cm−2, and a FF of 63.15%. This PCE value is comparable to the reported TPD and 2,5-di(thiophen-2-yl)thieno[3,2-b]thiophene (DT-TT) based polymers.35 Apparently, the Voc of P1 and P2 based solar cells was significantly larger than that of P3 based devices. This is consistent well with the deeper HOMO energy levels of the bi-TPD based polymers. The PCEs of P1 and P2 based devices may be further improved through device engineering similarly to that of PTB7-Th:PC71BM based solar cells.
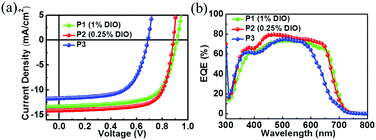 | ||
| Fig. 2 J–V curves (a) and the corresponding EQE curves (b) of the polymer:PC71BM solar cells with optimal performance. | ||
| Polymersa | V oc (V) | J sc (mA cm−2) | FF (%) | PCEb (%) |
|---|---|---|---|---|
a Blend ratios of P1:PC71BM (w/w) were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with 1% DIO, P2:PC71BM (w/w) were 1 2 with 1% DIO, P2:PC71BM (w/w) were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with 0.25% DIO and P3:PC71BM (w/w) was fixed at 1 2 with 0.25% DIO and P3:PC71BM (w/w) was fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 without DIO.
b In parentheses are average values based on more than 10 devices. 1.5 without DIO.
b In parentheses are average values based on more than 10 devices.
|
||||
| P1 | 0.91 | 13.45 | 67.18 | 8.22 (8.02 ± 0.20) |
| P2 | 0.89 | 14.15 | 72.13 | 9.08 (8.92 ± 0.16) |
| P3 | 0.69 | 11.70 | 63.15 | 5.10 (4.89 ± 0.21) |
The photo-response of the solar cells based on these polymers was investigated using their external quantum efficiencies (EQEs), and the EQE curves of the optimal devices are illustrated in Fig. 2b. The P1:PC71BM and P2:PC71BM devices showed a broader photo-response than the P3:PC71BM device, consistent with their thin film absorptions. The P2:PC71BM device exhibited the highest average EQE values among the three devices. The current density calculated from the EQE curves with a standard solar spectrum (AM 1.5G) was 13.25, 13.92, and 11.36 mA cm−2 for P1–P3, respectively, agreeing well with the ones obtained from J–V measurements.
To elucidate the observed performance of P1–P3 based solar cells, we explored the morphological features of the optimal blend films using transmission electron microscopy (TEM) and atomic force microscopy (AFM). Fig. 3 illustrates the TEM images of the blend films. All the films exhibited obvious dark and light domains, corresponding to the aggregates of PC71BM and the polymer, respectively. For P1 (P2):PC71BM films, nanofibrillar-like aggregates were formed and distributed throughout the blend films, resulting in an interpenetrating network. This kind of morphological feature is more favorable for efficient exciton separation and diffusion. P3:PC71BM films showed similar morphological features to the other two blend films. However, some aggregated structures of P3 (high brightness areas) were observed. AFM images revealed that P1:PC71BM films were comprised of large size grains with an RMS value of 4.21 nm (Fig. S5, ESI†). Compared to P1:PC71BM films, P2:PC71BM films exhibited smaller size domains and smoother surfaces with an RMS value of 1.73 nm. The RMS values of P3:PC71BM films were larger than 9 nm due to the formation of aggregated structures, which is consistent with the TEM results.
 | ||
| Fig. 3 TEM images of the polymer:PC71BM films: (a) P1:PC71BM; (b) P2:PC71BM; and (c) P3:PC71BM. The scale bars in the images are 200 nm. | ||
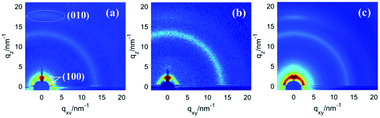
The crystallinity and molecular packing of the polymers in the active layers were probed using 2D grazing incidence X-ray diffraction (2D-GIXD) (Fig. 4). P1–P3 were crystalline and adopted edge-on and face-on co-orientations in the blend films, as proved by the (100) diffraction peaks in both the qxy-axis and the qz-axis. Moreover, π–π stacking diffractions (010 peaks) were observed in the qz-axis and the π–π stacking distances were calculated to be 3.52, 3.61, and 3.60 Å for P1, P2 and P3, respectively. The crystalline nature and co-existence of the edge-on and face-on packing structures of the films facilitated charge carrier transport in polymer solar cell, leading to high photovoltaic performance.
The hole and electron transport properties of the optimal polymer:PC71BM films in the direction perpendicular to the substrate were evaluated using space-charge-limited-current (SCLC) measurements. The J–V curves are shown in Fig. S6 (ESI†). For P1:PC71BM films, the calculated electron (μe) and hole (μh) mobilities were 4.48 × 10−4 cm2 V−1 s−1 and 1.99 × 10−4 cm2 V−1 s−1with a μe/μh ratio of 2.25. For P2:PC71BM films, the calculated μe and μh were 3.86 × 10−4 cm2 V−1 s−1 and 2.49 × 10−4 cm2 V−1 s−1 with a μe/μh ratio of 1.55. For P3:PC71BM films, the calculated μe and μh were 3.57 × 10−4 cm2 V−1 s−1 and 1.35 × 10−4 cm2 V−1 s−1 with a μh/μe ratio of 2.64. The relatively balanced carrier mobility of P2:PC71BM might be an important factor for its higher FF in solar cells.36
In conclusion, new bi-TPD based polymers P1 and P2 were designed and synthesized. Their physicochemical properties were investigated. Compared to their TPD-based analogue P3, P1 and P2 have deeper HOMO energy levels and exhibit red-shifted thin film UV-vis absorption spectra. The photovoltaic characteristics showed that cells based on bi-TPD based polymers P1 and P2 exhibited much higher PCE than that of mono-TPD based polymer P3. The optimal PCE is 8.22% for P1 cells, 9.08% for P2 cells and 5.10% for P3 cells. The PCE and FF values of P2 cells are among the highest values reported for single junction solar cells. The blend films of P1 (P2):PC71BM exhibited high crystallinity with face-on and edge-on co-orientations and good phase separation. These results demonstrate that bi-TPD is an excellent electron-deficient unit that is rarely investigated for high PCE polymer semiconductors. Through further material optimization in the side chains and donor units as well as device engineering, we believe, bi-TPD based polymers with higher efficiencies can be envisioned.
This work was supported by the National Natural Science Foundation of China (21474128, 21672252, 61405209, 21672130) and the “Strategic Priority Research Program” of the Chinese Academy of Sciences (XDB12010100). H. Li thanks the Shanghai Synchrotron Radiation Facility (Beamline BL14B1) for providing the beam time.
Notes and references
- L. Dou, Y. Liu, Z. Hong, G. Li and Y. Yang, Chem. Rev., 2015, 115, 12633 CrossRef CAS PubMed.
- L. Lu, T. Zheng, Q. Wu, A. M. Schneider, D. Zhao and L. Yu, Chem. Rev., 2015, 115, 12666 CrossRef CAS PubMed.
- F. C. Krebs, N. Espinosa, M. Hösel, R. R. Søndergaard and M. Jørgensen, Adv. Mater., 2014, 26, 29 CrossRef CAS PubMed.
- P. Cheng and X. Zhan, Chem. Soc. Rev., 2016, 45, 2544 RSC.
- Y. J. Cheng, S. H. Yang and C. S. Hsu, Chem. Rev., 2009, 109, 5868 CrossRef CAS PubMed.
- Y. Liu, J. Zhao, Z. Li, C. Mu, W. Ma, H. Hu, K. Jiang, H. Lin, H. Ade and H. Yan, Nat. Commun., 2014, 5, 5293 CrossRef CAS PubMed.
- W. Zhao, D. Qian, S. Zhang, S. Li, O. Inganas, F. Gao and J. Hui, Adv. Mater., 2016, 28, 4734 CrossRef CAS PubMed.
- H. Bin, L. Gao, Z. G. Zhang, Y. Yang, Y. Zhang, C. Zhang, S. Chen, L. Xue, C. Yang, M. Xiao and Y. Li, Nat. Commun., 2016, 7, 13651 CrossRef CAS PubMed.
- J. Chen, C. Cui, Y. Q. Li, L. Zhou, Q. D. Ou, C. Li, Y. Li and J. Tang, Adv. Mater., 2015, 27, 1035 CrossRef CAS PubMed.
- X. H. Ouyang, R. X. Peng, L. Ai, X. Y. Zhang and Z. Y. Ge, Nat. Photonics, 2015, 9, 520 CrossRef CAS.
- J. Zhang, Y. Zhang, J. Fang, K. Lu, Z. Wang, W. Ma and Z. Wei, J. Am. Chem. Soc., 2015, 137, 8176 CrossRef CAS PubMed.
- Y. Liu, Z. A. Page, T. P. Russell and T. Emrick, Angew. Chem., Int. Ed., 2015, 54, 11485 CrossRef CAS PubMed.
- Y. Li, Acc. Chem. Res., 2012, 45, 723 CrossRef CAS PubMed.
- J. W. Chen and Y. Cao, Acc. Chem. Res., 2009, 42, 1709 CrossRef CAS PubMed.
- M. Wang, D. Cai, Z. Yin, S. C. Chen, C. F. Du and Q. Zheng, Adv. Mater., 2016, 28, 3359 CrossRef CAS PubMed.
- K. Kawashima, T. Fukuhara, Y. Suda, Y. Suzuki, T. Koganezawa, H. Yoshida, H. Ohkita, I. Osaka and K. Takimiya, J. Am. Chem. Soc., 2016, 138, 10265 CrossRef CAS PubMed.
- H. Bin, Z. G. Zhang, L. Gao, S. Chen, L. Zhong, L. Xue, C. Yang and Y. Li, J. Am. Chem. Soc., 2016, 138, 4657 CrossRef CAS PubMed.
- R. S. Ashraf, I. Meager, M. Nikolka, M. Kirkus, M. Planells, B. C. Schroeder, S. Holliday, M. Hurhangee, C. B. Nielsen, H. Sirringhaus and I. McCulloch, J. Am. Chem. Soc., 2015, 137, 1314 CrossRef CAS PubMed.
- Y. Deng, J. Liu, J. Wang, L. Liu, W. Li, H. Tian, X. Zhang, Z. Xie, Y. Geng and F. Wang, Adv. Mater., 2014, 26, 471 CrossRef CAS PubMed.
- W. Chen, M. Xiao, L. Han, J. Zhang, H. Jiang, C. Gu, W. Shen and R. Yang, ACS Appl. Mater. Interfaces, 2016, 8, 19665 CAS.
- M. Zhang, Y. Gu, X. Guo, F. Liu, S. Zhang, L. Huo, T. P. Russell and J. Hou, Adv. Mater., 2013, 25, 4944 CrossRef CAS PubMed.
- J. You, L. Dou, K. Yoshimura, T. Kato, K. Ohya, T. Moriarty, K. Emery, C. C. Chen, J. Gao, G. Li and Y. Yang, Nat. Commun., 2013, 4, 1446 CrossRef PubMed.
- Z. He, B. Xiao, F. Liu, H. Wu, Y. Yang, S. Xiao, C. Wang, T. P. Russell and Y. Cao, Nat. Photonics, 2015, 9, 174 CrossRef CAS.
- M. Zhang, X. Guo, S. Zhang and J. Hou, Adv. Mater., 2014, 26, 1118 CrossRef CAS PubMed.
- Y. Zou, A. Najari, P. Berrouard, S. Beaupré, B. R. Aïch, Y. Tao and M. Leclerc, J. Am. Chem. Soc., 2010, 132, 5330 CrossRef CAS PubMed.
- E. Zhou, J. Cong, K. Tajima, C. Yang and K. Hashimoto, Macromol. Chem. Phys., 2011, 212, 305 CrossRef CAS.
- E. Zhou, J. Cong, K. Tajima, C. Yang and K. Hashimoto, J. Phys. Chem. C, 2012, 116, 2608 CAS.
- J. W. Jung, J. W. Jo, E. H. Jung and W. H. Jo, Org. Electron., 2016, 31, 149 CrossRef CAS.
- P. Berrouard, F. Grenier, J. R. Pouliot, E. Gagnon, C. Tessier and M. Leclerc, Org. Lett., 2011, 13, 38 CrossRef CAS PubMed.
- J. E. Donaghey, R. S. Ashraf, Y. Kim, Z. G. Huang, C. B. Nielsen, W. Zhang, B. Schroeder, C. R. G. Grenier, C. T. Brown, P. D'Angelo, J. Smith, S. Watkins, K. Song, T. D. Anthopoulos, J. R. Durrant, C. K. Williamsa and I. McCullocha, J. Mater. Chem., 2011, 21, 18744 RSC.
- X. Qiao, Q. Wu, H. Wu, D. Wang and H. Li, Polym. Chem., 2016, 7, 807 RSC.
- B. Carsten, J. M. Szarko, H. J. Son, W. Wang, L. Lu, F. He, B. S. Rolczynski, S. J. Lou, L. X. Chen and L. Yu, J. Am. Chem. Soc., 2011, 133, 20468 CrossRef CAS PubMed.
- R. S. Bhatta and M. Tsige, ACS Appl. Mater. Interfaces, 2014, 6, 15889 CAS.
- Q. Wu, M. Wang, X. Qiao, Y. Xiong, Y. Huang, X. Gao and H. Li, Macromolecules, 2013, 46, 3887 CrossRef CAS.
- G. Y. Chen, Y. H. Cheng, Y. J. Chou, M. S. Su, C. M. Chen and K. H. Wei, Chem. Commun., 2011, 47, 5064 RSC.
- G. Li, V. Shrotriya, J. S. Huang, Y. Yao, T. Moriarty, K. Emery and Y. Yang, Nat. Mater., 2005, 4, 864 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis of the polymers, device fabrication and characterization, cyclic voltammetry, J–V curves and photovoltaic parameters of PSCs based on polymer:PC71BM, AFM images of the blend films and J–V curves of vertical diodes for hole (electron) only devices. See DOI: 10.1039/c7cc00501f |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2017 |