Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rapid detection of nicotine from breath using desorption ionisation on porous silicon†
T. M.
Guinan
 ab,
H.
Abdelmaksoud
ab and
N. H.
Voelcker‡
ab,
H.
Abdelmaksoud
ab and
N. H.
Voelcker‡
 *ab
*ab
aFuture Industries Institute, University of South Australia, Adelaide, South Australia, Australia
bMonash Institute of Pharmaceutical Sciences, Monash University, Parkville, Victoria, Australia
First published on 19th April 2017
Abstract
Desorption ionisation on porous silicon (DIOS) was used for the detection of nicotine from exhaled breath. This result represents proof-of-principle of the ability of DIOS to detect small molecular analytes in breath including biomarkers and illicit drugs.
Matrix-assisted laser desorption ionisation mass spectrometry (MALDI-MS) is a technique capable of high-throughput analysis often used for the detection of peptides,1 proteins2 and oligonucleotides. MALDI-MS involves the co-crystallisation of a UV absorbing matrix and sample on a conductive surface.3 Subsequently, a pulsed UV laser is used to facilitate the simultaneous desorption/ionisation of the analyte and matrix. However, this technique is not conducive to small molecule analysis due to the matrix and its fragment peaks obscuring the low mass range typically below 700 Da.4 Surface-assisted laser desorption ionisation mass spectrometry (SALDI-MS) is an adaption of MALDI-MS and employs nanostructured surfaces with UV absorbing properties to alleviate the need for the matrix. In 1999, Suizdak et al. developed one of the first representations of SALDI which was termed desorption ionisation on porous silicon (DIOS).5 DIOS chips utilise nanostructured porous silicon (pSi) due to its high surface area, inherent UV absorptivity and ease of functionalisation.6 Nanostructured pSi is fabricated using a light-assisted anodic etch in hydrofluoric acid.7 DIOS has been used previously for the simultaneous detection of a range of small molecules including illicit drugs from saliva,7,8 plasma,9 urine,9 and sweat.10 Quantification with DIOS has been routinely demonstrated with detection limits in the order of nanogram per millilitres.11 Point of collection drug testing using non-invasive biological fluids has been introduced in a range of settings including for roadside,12 workplace,13 drug compliance14 and athlete screening.15 For example, roadside drug testing legislation has been introduced in many countries including Australia since the early 2000's.16 The current procedure involves the immunoassay based screening of saliva for methamphetamine (MA), 3,4-methylenedioxymethamphetamine (MDMA) and tetrahydrocannabinol (THC).17 However, these techniques are presumptive in nature, suffer from cross-reactivity and often give false positives/negatives.18 As a result, additional laboratory testing using gas chromatography mass spectrometry (GC-MS) or liquid chromatography-mass spectrometry (LC-MS) is required off-site, significantly delaying the process of conviction or acquittal.
Breath testing is a powerful technique, which holds promise in the field of drug detection,19 and biomarker discovery.20 Exhaled breath is composed of molecules, which are trapped in aerosol particles formed from the airway lining fluid. Breath analysis offers a rapid, unalterable and non-invasive means of testing, which is globally accepted for point of collection alcohol testing.21 Recently, several studies have emerged which demonstrate the detection of drugs of abuse from breath is also possible using LC-MS.19 Furthermore, nicotine has been detected from vapour using various MS approaches.22,23 The current validated procedure employs a breath collection device with a filter. The filter is designed to trap the aerosol particles containing drug molecules but the drugs must then be extracted and concentrated from the filter for mass spectrometry analysis.19
Here, we demonstrate the DIOS-MS detection of nicotine from breath using two different facile protocols. Unlike current mass spectrometry based techniques, our novel approach allows for direct detection of small molecules without the need for extraction, derivatisation or rinsing protocols. This technique also rules out the possibility of adulteration since breath samples can be taken directly by the analyser. Breath capture and processing was optimised for each protocol with factors including resuspension volume assessed. Furthermore, MS/MS was used to confirm that detection of nicotine. The signal-to-noise (S/N) was analysed over time for a smoker. Finally, the breath of a non-smoker was analysed to further confirm the successful detection of nicotine.
Scanning electron micrographs (SEM) of DIOS chips with 101 ± 19 nm pore diameter and 660 nm pore depth are displayed in Fig. S1A and B (ESI†), respectively. The DIOS chips were functionalised with (tridecafluoro-1,1,2,2-tetrahydrooctyl)-dimethylchlorosilane (F13) as described previously.24
Prior to breath testing studies, the analytical sensitivity of DIOS-MS was assessed for the detection of nicotine in water for the concentration range (0–10 ng mL−1, Fig. 1). The limit of detection (LOD) was defined as three standard deviations of the baseline.25 The baseline was calculated from the average S/N for six replicates from a blank sample containing no nicotine. Subsequently, the LOD was determined to be 0.54 ng mL−1 for nicotine in water, and good linearity (R2 > 0.994) was observed.
 | ||
| Fig. 1 Linear regression for nicotine analysed in milliQ water using DIOS. Inset shows DIOS-MS for the analysis of nicotine (ion of m/z 163) in water at 10 ng mL−1. Additional background peaks commonly observed on DIOS at m/z 130 are also detected.11 | ||
Fig. 1 (inset) displays representative DIOS-MS for nicotine from an analytical standard at 10 ng mL−1 with a high intensity ion of m/z 163 observed.
Breath capture methods were next trialled to optimise the S/N for nicotine detection from breath. The first protocol involved the direct exhalation of breath for 15 s onto a DIOS chip inserted in a straw (Fig. S2, ESI†), whereas the second protocol involved exhalation into an Eppendorf (Fig. S2, ESI†) and subsequent resuspension using varying volumes of milliQ water (5–20 μL). Representative DIOS-MS from protocol 1 and 2 for non-smoker and smoker breath samples are shown in Fig. S3A and B (ESI†), respectively. Indeed, an abundant ion of m/z 163 was observed for both protocols from the exhaled breath of a habitual smoker.
A low signal intensity at m/z 163 was also observed for control samples which was due to an isotopic peak of an unknown background or breath related compound observed at an ion of m/z 162.
The identity of this peak could not be confirmed using DIOS-MS/MS due to the low signal intensity. The observed S/N at m/z 163 was less than 24 for the exhaled breath of the non-smoker for each protocol and was statistically different from the S/N of smoker breath (>100). DIOS-MS/MS from protocol 2 was used to confirm the identity of nicotine from the exhaled breath of the smoker since it produced the highest overall S/N (Fig. 2). Fragment peaks at ions of m/z 132, 120, 102 and 86, respectively, were observed in good agreement with the literature.26
Fig. 3 displays a comparison of the performance for the two protocols where all breath samples were taken from the subject (smoker or non-smoker) consecutively in no particular order. Protocol 1 was performed on three separate DIOS sections (0.5 cm × 1.5 cm) and protocol 2 involved the deposition of 1 μL of resuspended breath onto a 2.5 × 2.5 cm DIOS chip. For each protocol, excellent reproducibility was observed from sample-to-sample (protocol 1) and spot-to-spot (protocol 2). Since protocol 2 involves the resuspension of breath in water (added after exhaling) the protocol was optimised in terms of resuspension volume. The S/N for the 5 μL volume was observed to be 3.2, 4.5 and 6.8 times higher for the 10, 20 μL and protocol 1, respectively. This observed increase in S/N is due the lower resuspension volume (5 μL) acting like a preconcentration step for nicotine. However, volumes less than 5 μL were not trialled for protocol 2 because at least three replicates are required for quantitative analysis. Protocol 2 was preferred for the final analysis due to observed higher S/N compared to protocol 1. The observed increase in signal is likely due to the breath being confined in the Eppendorf and then pipetted onto the DIOS chip in 1 μL aliquots, whereas the breath in protocol 1 will be “spread” over the DIOS substrate. Furthermore, protocol 2 allows for storage of samples, multiple replicates and ease of transport for future analysis.
 | ||
| Fig. 3 Average S/N observed for protocol 1 and 2, respectively, with error bars corresponding to standard deviation (n = 3). For protocol 2, three resuspension volumes were compared for a smoker. | ||
Nicotine is observed in low concentrations in the blood after smoking (1–15 ng mL−1) and has a half life in blood plasma of approximately 1–2 h.27
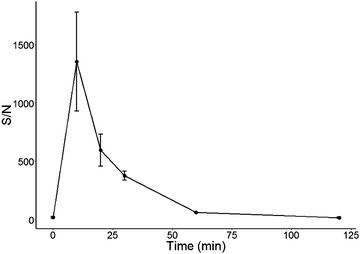
Fig. 4 displays the observed S/N for nicotine using protocol 2 (5 μL resuspension volume), from 0–120 min from the exhaled breath of a smoker. The time point 0 min corresponds to the breath taken from the participant immediately prior to smoking after not smoking a cigarette for a period of 12 h. Indeed, the S/N (approx. S/N of 22) observed for this time point was in line with S/N values observed for the control participant (Fig. 3, approx. S/N of 24). A peak concentration was observed 10 min after the participant had smoked, which was then observed to decrease over time. After 120 min, an average S/N of 18 was observed indicating that nicotine was no longer present in breath. These results correlate well with blood plasma concentrations observed for nicotine from cigarettes.27
In summary, DIOS-MS has been utilised for the detection of nicotine directly from breath. Our approach allows for non-invasive sampling without the possibility of adulteration and therefore has the possibility to replace other body fluids as a testing fluid of choice. Furthermore, DIOS-MS has been previously demonstrated to be capable of simultaneous analyte detection28 and therefore may be useful in drugs of abuse and biomarker detection. This facile approach may engender high impact applications in the field of drug detection from breath for workplace, roadside and airport testing. Furthermore, we believe that this unique DIOS-MS approach for breath analysis may also allow for biomarker discovery in the field of cancer diagnostics.
This research was conducted and funded by the Australian Research Linkage project (Project No. LP110200446). We would like to acknowledge Peter Stockham at Forensic Science South Australia for helpful discussions and kindly providing nicotine standards.
Notes and references
- F. Hillenkamp, T. W. Jaskolla and M. Karas, MALDI MS, Wiley-VCH Verlag GmbH & Co. KGaA, 2013, pp. 1–40, DOI:10.1002/9783527335961.ch1.
- V. Mainini, G. Bovo, C. Chinello, E. Gianazza, M. Grasso, G. Cattoretti and F. Magni, Mol. BioSyst., 2013, 9, 1101–1107 RSC.
- K. Dreisewerd, Chem. Rev., 2003, 103, 395–425 CrossRef CAS PubMed.
- J. J. Thomas, Z. Shen, R. Blackledge and G. Siuzdak, Anal. Chim. Acta, 2001, 442, 183–190 CrossRef CAS.
- J. Wei, J. M. Buriak and G. Siuzdak, Nature, 1999, 399, 243–246 CrossRef CAS PubMed.
- T. Guinan, P. Kirkbride, P. E. Pigou, M. Ronci, H. Kobus and N. H. Voelcker, Mass Spectrom. Rev., 2015, 34, 627–640 CrossRef CAS PubMed.
- T. Guinan, M. Ronci, H. Kobus and N. H. Voelcker, Talanta, 2012, 99, 791–798 CrossRef CAS PubMed.
- T. M. Guinan, P. Kirkbride, C. B. Della Vedova, S. G. Kershaw, H. Kobus and N. H. Voelcker, Analyst, 2015, 140, 7926–7933 RSC.
- T. M. Guinan, D. Neldner, P. Stockham, H. Kobus, C. B. Della Vedova and N. H. Voelcker, Drug Test. Anal., 2016 DOI:10.1002/dta.2033.
- T. Guinan, C. Della Vedova, H. Kobus and N. H. Voelcker, Chem. Commun., 2015, 51, 6088–6091 RSC.
- T. Guinan, M. Ronci, R. Vasani, H. Kobus and N. H. Voelcker, Talanta, 2015, 132, 494–502 CrossRef CAS PubMed.
- G. Skopp and L. Pötsch, Int. J. Leg. Med., 1999, 112, 213–221 CrossRef CAS PubMed.
- G. Cooper, C. Moore, C. George and S. Pichini, Drug Test. Anal., 2011, 3, 269–276 CrossRef CAS PubMed.
- M. E. Larson and T. M. Richards, Clin. Med. Res., 2009, 7, 134–141 CrossRef CAS PubMed.
- P. Schamasch and O. Rabin, Bioanalysis, 2012, 4, 1691–1701 CrossRef CAS PubMed.
- A. Verstraete and L. Labat, Ann. Toxicol. Anal., 2009, 21, 3–8 CrossRef CAS.
- O. H. Drummer, Ther. Drug Monit., 2008, 30, 203–206 CAS.
- A. Verstraete and E. Raes, Rosita-2 project, 2006, 189.
- N. Stephanson, S. Sandqvist, M. S. Lambert and O. Beck, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2015, 985, 189–196 CrossRef CAS PubMed.
- J. D. Beauchamp, EBioMedicine, 2015, 2, 1030–1031 CrossRef PubMed.
- R. F. Borkenstein and H. Smith, Med., Sci. Law, 1961, 2, 13–22 CrossRef CAS.
- Y. Liu, S. Antwi-Boampong, J. J. Bel Bruno, M. A. Crane and S. E. Tanski, Nicotine Tob. Res., 2013, 15, 1511–1518 CrossRef CAS PubMed.
- M. Li, J. Ding, H. Gu, Y. Zhang, S. Pan, N. Xu, H. Chen and H. Li, Sci. Rep., 2013, 3, 1205 CrossRef PubMed.
- T. M. Guinan, O. J. R. Gustafsson, G. McPhee, H. Kobus and N. H. Voelcker, Anal. Chem., 2015, 87, 11195–11202 CrossRef CAS PubMed.
- H. Z. Alhmoud, T. M. Guinan, R. Elnathan, H. Kobus and N. H. Voelcker, Analyst, 2014, 139, 5999–6009 RSC.
- G. D. Byrd, R. A. Davis and M. W. Ogden, J. Chromatogr. Sci., 2005, 43, 133–140 CAS.
- N. L. Benowitz and P. Jacob, Clin. Pharmacol. Ther., 1993, 53, 316–323 CrossRef CAS PubMed.
- R. D. Lowe, G. Guild, P. Harpas, G. Siuzdak, P. Kirkbride, P. Hoffman, N. H. Voelcker and H. Kobus, Rapid Commun. Mass Spectrom., 2009, 23, 3543–3548 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional mass spectra, schematics and experimental methods. See DOI: 10.1039/c7cc00243b |
| ‡ Current address: Monash Institute of Pharmaceutical Sciences, Monash University, 381 Royal Parade, VIC 3052, Australia. E-mail: Nicolas.voelcker@monash.edu; Fax: +61 3 9903 9581. |
| This journal is © The Royal Society of Chemistry 2017 |