Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
UV-light promoted C–H bond activation of benzene and fluorobenzenes by an iridium(I) pincer complex†
Simone A.
Hauser
,
Jack
Emerson-King
,
Scott
Habershon
and
Adrian B.
Chaplin
 *
*
Department of Chemistry, University of Warwick, Gibbet Hill Road, Coventry CV4 7AL, UK. E-mail: a.b.chaplin@warwick.ac.uk; Web: http://go.warwick.ac.uk/abchaplin Web: www.twitter.com/chaplinlab
First published on 17th March 2017
Abstract
Iridium(I) carbonyl complex [Ir(2,6-(PtBu2CH2)2C6H3)(CO)] undergoes reversible C–H bond activation of benzene and a series of fluorobenzenes on UV irradiation. Exclusive ortho-selectivity is observed in reactions of fluorobenzene and 1,2-difluorobenzene.
Epitomised by applications in the catalytic dehydrogenation of alkanes, iridium complexes of phosphine-based pincer ligands are widely recognised for their capacity to activate C–H bonds.1 With fluoroaryls representing valuable synthons in organic chemistry,2,3 we have targeted use of these iridium compounds for carrying out selective C–H bond activation reactions of partially fluorinated benzenes (C6H6−nFn, n ≤ 3).3 The presence of fluorine substituents results in significantly stronger C–H bonds than benzene and, correspondingly, fluorobenzenes represent challenging substrates.3,4 Previous work by Milstein and Ozerov employing neutral (PNP) and anionic (PNP*) pincer ligands has highlighted the potential of iridium pincers, although under moderate temperature regimes (<100 °C) these systems showed poor regioselectivity in the activation of fluorobenzene (Scheme 1).5 As C–H bond activation is thermodynamically favoured ortho to the fluorine substituents,4 indiscriminate and irreversible oxidative addition reactions of transient 14 VE Ir(I) intermediates {Ir(PNP)}+/{Ir(PNP*)} are implicated.
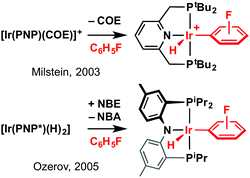 | ||
| Scheme 1 C–H bond activation of fluorobenzene using Ir-pincers. PNP = 2,6-(PtBu2CH2)2C5H3N, PNP* = (4-Me-2-(iPr2P)C6H3)2N−, COE = cyclooctene, NBE = norbornene, NBA = norbornane. | ||
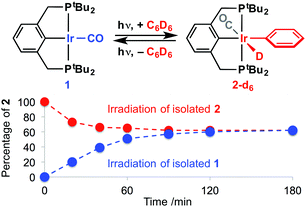
We postulated that use of anionic pincer ligands bearing central aryl donors could promote selective activation of fluorobenzene substrates through increased reaction reversibility imparted by the high trans-influence aryl donor.6,7 With a view to testing this hypothesis we selected [Ir(PCP)(CO)] 1 (PCP = 2,6-(PtBu2CH2)2C6H3−)8 as a well-defined precursor for the low coordinate and formally 14 VE Ir(I) fragment {Ir(PCP)}, through photochemically promoted dissociation of the carbonyl ligand. In this way, subsequent products of C–H bond activation would be trapped on re-coordination of the carbonyl ligand (in a closed system). Initial experiments using benzene as the substrate supported this reasoning, with [Ir(PCP)(C6D5)D(CO)] 2-d69 generated on irradiation of a 20 mM C6D6 solution of 1 at RT using a 100 W Hg arc lamp (quartz J. Young's NMR tube, Scheme 2). Following this reaction by periodic analysis using 31P NMR spectroscopy, however, indicated that conversion of 1 (δ31P 82.0) to 2-d6 (δ31P 52.3) plateaued at 62% after ca. 2 h total irradiation. On the same timeframe, irradiation of independently synthesised 2 resulted in an equivalent reaction composition. In contrast, both 1 and 2 are thermally stable on extended heating at 80 °C in C6D6 solution (8 h) and no isotope exchange was observed for 2 (to 2-d6).10 Together these results indicate establishment of a photostationary mixture of 1 and 2-d6, mediated through light induced carbonyl dissociation from both species.11
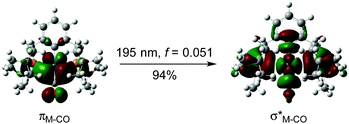
To gain deeper understanding of the photolysis experiments, a series of DFT and TD-DFT calculations were performed (see ESI† for full details). In line with expectation, the computed free energy for carbonyl dissociation to form {Ir(PCP)} is a significantly endergonic process (ΔG298K = +194.4 kJ mol−1). While the subsequent C–H bond activation of benzene is exothermic (ΔH = −15.3 kJ mol−1), the Ir(III) product lies thermodynamically further uphill from 1 (ΔG298K = +238.0 kJ mol−1). Such energetics are characteristic of an unfavourable equilibrium reaction, although one that would be offset by the use of the substrate as the solvent.12 Re-coordination of the carbonyl ligand counteracts the unfavourable thermodynamics (ΔG298K = −95.0 kJ mol−1), however, 2 is still calculated to be +141.3 kJ mol−1 higher in free energy than 1 + C6H6. Together these results are consistent with the lack of any thermal reaction observed for either 1 or 2 in C6D6 and highlight the important promoting role of UV-irradiation in the formation of 2 (and reformation of 1). In this context, analysis of 1 by TD-DFT identified a number of singlet–singlet electronic transitions between 195 and 235 nm (i.e. UV) that can be attributed to carbonyl dissociation. A representative example is shown in Fig. 1 (full details provided in ESI†). Similar excitations are also calculated for 2 between 190 and 260 nm, suggesting that it would be difficult to selectively enact the UV-promoted dissociation of carbonyl from either 1 or 2.13
To further explore this C–H bond activation chemistry, 20 mM solutions of 1 in fluorobenzene, 1,2-difluorobenzene, and 1,3,5-trifluorobenzene were irradiated for a total of 8 h at RT (Scheme 3). Similar to that observed in benzene, analysis by 1H and 31P NMR spectroscopy indicated formation of photostationary mixtures composed of 1 and Ir(III) products of C–H bond activation 3; as the minor and major components, respectively. Supporting our hypothesis, and contrasting with reactions of analogous PNP and PNP* systems (vide supra), C–H bond activation of fluorobenzene (3a) and 1,2-difluorobenzene (3b) proceeded with exclusive ortho-selectivity. Both possible rotamers of each isomer are formed, although in disparate proportions (Scheme 3).
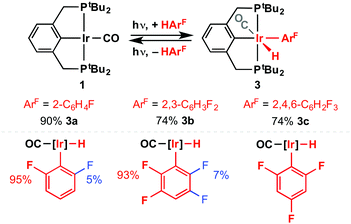 | ||
| Scheme 3 UV-promoted C–H bond activation of fluorobenzenes (top; [Ir] = 20 mM, 8 h, RT). Major rotamers depicted in red, minor rotamers depicted in blue (bottom). | ||
Consistent with these observations and manifestation of the “ortho fluorine effect”,4 the alternative regioisomers of 3a (>10 kJ mol−1) and 3b (>18 kJ mol−1), and their respective 5-coordinate precursors [Ir(PCP)(2-FC6H4)H] (>24 kJ mol−1) and [Ir(PCP)(2,3-F2C6H3)H] (>26 kJ mol−1), are calculated to be significantly higher in free energy. As for the formation of 2 from 1, computed reaction free energies suggest the formation of 3 occur outside of thermally accessible regimes and instead are presumably driven through nuanced differences in the photophysical properties of 1 and 3. Moreover, increasingly favourable overall reactions correlate with number of fluorine substituents: ΔG298K/kJ mol−1 = +141.3 (2), +128.1 (3a), +121.0 (3b), +119.1 (3c).
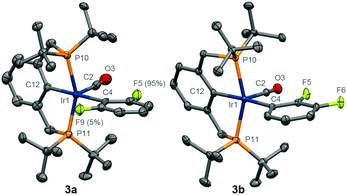
With the above in mind and to independently verify their structures, analytically pure samples of 3 were isolated though dehydrohalogenation of [Ir(PCP)HCl]14 using K[OtBu] in the respective fluorobenzene at 75 °C, followed by reaction with carbon monoxide (yield = 32–54%). The structures of these new compounds, and for comparison 2,9 were fully verified in solution by 1H, 13C, 19F and 31P NMR, IR and UV-vis spectroscopy, and in the solid-state by X-ray crystallography (3a, 3b – Fig. 2; 2, 3c – ESI†). Notable spectroscopic markers include low frequency hydride resonances at δ −9.07 (2), −8.86/−![[9 with combining low line]](https://www.rsc.org/images/entities/char_0039_0332.gif)
![[. with combining low line]](https://www.rsc.org/images/entities/char_002e_0332.gif)
![[6 with combining low line]](https://www.rsc.org/images/entities/char_0036_0332.gif)
![[8 with combining low line]](https://www.rsc.org/images/entities/char_0038_0332.gif) (3a), −8.88/−
(3a), −8.88/−![[9 with combining low line]](https://www.rsc.org/images/entities/char_0039_0332.gif)
![[. with combining low line]](https://www.rsc.org/images/entities/char_002e_0332.gif)
![[6 with combining low line]](https://www.rsc.org/images/entities/char_0036_0332.gif)
![[6 with combining low line]](https://www.rsc.org/images/entities/char_0036_0332.gif) (3b), −9.46 (3c) that show 3JPH coupling of ca. 17 Hz, and 31P resonances at δ 51.1 (2), 54.5/
(3b), −9.46 (3c) that show 3JPH coupling of ca. 17 Hz, and 31P resonances at δ 51.1 (2), 54.5/![[5 with combining low line]](https://www.rsc.org/images/entities/char_0035_0332.gif)
![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif)
![[. with combining low line]](https://www.rsc.org/images/entities/char_002e_0332.gif)
![[7 with combining low line]](https://www.rsc.org/images/entities/char_0037_0332.gif) (3a), 54.7/
(3a), 54.7/![[5 with combining low line]](https://www.rsc.org/images/entities/char_0035_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif)
![[. with combining low line]](https://www.rsc.org/images/entities/char_002e_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) (3b), 54.0 (3c) in C6D12 solution (data for major rotamers underlined). The structures of major rotamers of 3a and 3b, bearing the fluorine atoms proximal to the carbonyl ligand, were definitively established in solution through NOESY experiments and corroborated in the solid-state (Fig. 2). Likewise, the hydride resonances of the minor rotamers show characteristic 1hJFH coupling of ca. 10 Hz. The observed relative conformational preferences are also reproduced in silico (see ESI†). Complexes 3 are thermally stable on extended heating at 80 °C in C6D6 solution: after 8 h, <5% 1 is formed and for 3a and 3b the ratio of rotamers was unchanged, as determined in situ by 31P NMR spectroscopy.
(3b), 54.0 (3c) in C6D12 solution (data for major rotamers underlined). The structures of major rotamers of 3a and 3b, bearing the fluorine atoms proximal to the carbonyl ligand, were definitively established in solution through NOESY experiments and corroborated in the solid-state (Fig. 2). Likewise, the hydride resonances of the minor rotamers show characteristic 1hJFH coupling of ca. 10 Hz. The observed relative conformational preferences are also reproduced in silico (see ESI†). Complexes 3 are thermally stable on extended heating at 80 °C in C6D6 solution: after 8 h, <5% 1 is formed and for 3a and 3b the ratio of rotamers was unchanged, as determined in situ by 31P NMR spectroscopy.
In conclusion, we have demonstrated that selective C–H bond activation reactions of fluorobenzenes can be achieved under mild conditions using photolysis of [Ir(2,6-(PtBu2CH2)2C6H3)(CO)] 1 as a means to generate the reactive 14 VE Ir(I) fragment {Ir(2,6-(PtBu2CH2)2C6H3)} in solution. This work not only showcases the ability of iridium pincer complexes to mediate challenging C–H bond activations, but more generally highlights a potentially useful catalyst design principle that we hope will stimulate the development of new organic transformations employing partially fluorinated benzenes as building blocks.
We gratefully acknowledge financial support from the Swiss National Science Foundation (S. A. H.), EPSRC (J. E.-K.), Royal Society (A. B. C.) and University of Warwick. Crystallographic data was collected using a diffractometer purchased through support from Advantage West Midlands and the European Regional Development Fund. We also thank the Scientific Computing Research Technology Platform at the University of Warwick for providing high-performance computing facilities.
Notes and references
- (a) K. Krogh-Jespersen, M. Czerw, K. Zhu, B. Singh, M. Kanzelberger, N. Darji, P. D. Achord, K. B. Renkema and A. S. Goldman, J. Am. Chem. Soc., 2002, 124, 10797–10809 CrossRef CAS PubMed; (b) R. Ghosh, X. Zhang, P. Achord, T. J. Emge, K. Krogh-Jespersen and A. S. Goldman, J. Am. Chem. Soc., 2007, 129, 853–866 CrossRef CAS PubMed; (c) W. H. Bernskoetter, P. S. White, K. I. Goldberg and M. Brookhart, J. Am. Chem. Soc., 2009, 131, 8603–8613 CrossRef CAS PubMed; (d) J. Choi, A. H. R. MacArthur, M. Brookhart and A. S. Goldman, Chem. Rev., 2011, 111, 1761–1779 CrossRef CAS PubMed; (e) J. Choi, D. Y. Wang, S. Kundu, Y. Choliy, T. J. Emge, K. Krogh-Jespersen and A. S. Goldman, Science, 2011, 332, 1545–1548 CrossRef CAS PubMed.
- For representative examples see: (a) M. Lafrance, C. N. Rowley, T. K. Woo and K. Fagnou, J. Am. Chem. Soc., 2006, 128, 8754–8756 CrossRef CAS PubMed; (b) H.-Q. Do and O. Daugulis, J. Am. Chem. Soc., 2008, 130, 1128–1129 CrossRef CAS PubMed; (c) Y. Nakao, N. Kashihara, K. S. Kanyiva and T. Hiyama, J. Am. Chem. Soc., 2008, 130, 16170–16171 CrossRef CAS PubMed; (d) X. C. Cambeiro, T. C. Boorman, P. Lu and I. Larrosa, Angew. Chem., Int. Ed., 2012, 52, 1781–1784 CrossRef PubMed; (e) P. Ricci, K. Krämer, X. C. Cambeiro and I. Larrosa, J. Am. Chem. Soc., 2013, 135, 13258–13261 CrossRef CAS PubMed; (f) X. C. Cambeiro, N. Ahlsten and I. Larrosa, J. Am. Chem. Soc., 2015, 137, 15636–15639 CrossRef CAS PubMed; (g) J. Takaya, S. Ito, H. Nomoto, N. Saito, N. Kirai and N. Iwasawa, Chem. Commun., 2015, 51, 17662–17665 RSC; (h) M. Simonetti, G. J. P. Perry, X. C. Cambeiro, F. Juliá-Hernández, J. N. Arokianathar and I. Larrosa, J. Am. Chem. Soc., 2016, 138, 3596–3606 CrossRef CAS PubMed.
- (a) E. Clot, O. Eisenstein, N. Jasim, S. A. Macgregor, J. E. McGrady and R. N. Perutz, Acc. Chem. Res., 2011, 44, 333–348 CrossRef CAS PubMed; (b) S. D. Pike, M. R. Crimmin and A. B. Chaplin, Chem. Commun., 2017, 53 10.1039/c6cc09575e.
- (a) M. E. Evans, C. L. Burke, S. Yaibuathes, E. Clot, O. Eisenstein and W. D. Jones, J. Am. Chem. Soc., 2009, 131, 13464–13473 CrossRef CAS PubMed; (b) E. Clot, C. Mégret, O. Eisenstein and R. N. Perutz, J. Am. Chem. Soc., 2009, 131, 7817–7827 CrossRef CAS PubMed; (c) E. Clot, M. Besora, F. Maseras, C. Mégret, O. Eisenstein, B. Oelckers and R. N. Perutz, Chem. Commun., 2003, 490–491 RSC.
- (a) L. Fan, S. Parkin and O. V. Ozerov, J. Am. Chem. Soc., 2005, 127, 16772–16773 CrossRef CAS PubMed; (b) E. Ben-Ari, M. Gandelman, H. Rozenberg, L. J. W. Shimon and D. Milstein, J. Am. Chem. Soc., 2003, 125, 4714–4715 CrossRef CAS PubMed; (c) E. Ben-Ari, R. Cohen, M. Gandelman, L. J. W. Shimon, J. M. L. Martin and D. Milstein, Organometallics, 2006, 25, 3190–3210 CrossRef CAS.
- D. Y. Wang, Y. Choliy, M. C. Haibach, J. F. Hartwig, K. Krogh-Jespersen and A. S. Goldman, J. Am. Chem. Soc., 2016, 138, 149–163 CrossRef CAS PubMed.
- During the preparation of this manuscript the C–H bond activation of fluorobenzene using related Ir-POCOP pincer complexes has been described: L. P. Press, A. J. Kosanovich, B. J. McCulloch and O. V. Ozerov, J. Am. Chem. Soc., 2016, 138, 9487–9497 CrossRef PubMed.
- (a) K. Krogh-Jespersen, M. Czerw, K. Zhu, B. Singh, M. Kanzelberger, N. Darji, P. D. Achord, K. B. Renkema and A. S. Goldman, J. Am. Chem. Soc., 2002, 124, 10797–10809 CrossRef CAS PubMed; (b) D. Morales-Morales, R. Redón, Z. Wang, Do W. Lee, C. Yung, K. Magnuson and C. M. Jensen, Can. J. Chem., 2001, 79, 823–829 CrossRef CAS.
- M. Kanzelberger, B. Singh, M. Czerw, K. Krogh-Jespersen and A. S. Goldman, J. Am. Chem. Soc., 2000, 122, 11017–11018 CrossRef CAS.
- Acid catalysed oxidative addition of C(sp)–H bonds to 1 have, however, been reported: J. D. Hackenberg, S. Kundu, T. J. Emge, K. Krogh-Jespersen and A. S. Goldman, J. Am. Chem. Soc., 2014, 136, 8891–8894 CrossRef CAS PubMed.
- Consistent with this suggestion, photolysis of 20 mM solutions of 1 and 2 in C6D6 under 1 atm of CO at RT resulted in prolonged (>6 h) establishment of similar photostationary mixtures of 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-d6 (1:
2-d6 (1:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4, corresponding to 58% 2-d6) to those obtained under an argon atmosphere (after ca. 2 h). For discussion of mechanistic pathways in related photochemical reactions of [Rh(PMe3)2(CO)Cl] see: G. P. Rosini, W. T. Boese and A. S. Goldman, J. Am. Chem. Soc., 1994, 116, 9498–9505 CrossRef CAS.
1.4, corresponding to 58% 2-d6) to those obtained under an argon atmosphere (after ca. 2 h). For discussion of mechanistic pathways in related photochemical reactions of [Rh(PMe3)2(CO)Cl] see: G. P. Rosini, W. T. Boese and A. S. Goldman, J. Am. Chem. Soc., 1994, 116, 9498–9505 CrossRef CAS. - J. E. Wheatley, C. A. Ohlin and A. B. Chaplin, Chem. Commun., 2014, 50, 685–687 RSC.
- Notably using a long pass (glass) filter (<330 nm): no reaction was observed when a 20 mM solution of 1 was irradiated in C6D6 for 2 h at RT, while <5% formation of 1 was observed when a 20 mM solution of 2 was irradiated in C6D6 for 2 h at RT.
- C. J. Moulton and B. L. Shaw, J. Chem. Soc., Dalton Trans., 1976, 1020 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental and computational details, NMR, IR and UV-vis spectra, TD-DFT analysis of 1 and 2, and optimised geometries in xyz format. CCDC 1520522–1520525. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc09807j |
| This journal is © The Royal Society of Chemistry 2017 |