Open Access Article
Open Access Articleαvβ3-Isoform specific erbium complexes highly specific for bladder cancer imaging and photodynamic therapy†
Yan
Zhou
a,
Chi-Fai
Chan
b,
Daniel W. J.
Kwong
*a,
Ga-Lai
Law
*b,
Steven
Cobb
c,
Wai-Kwok
Wong
*a and
Ka-Leung
Wong
 *a
*a
aDepartment of Chemistry, Hong Kong Baptist University, Kowloon Tong, Hong Kong SAR, China. E-mail: wkwong@hkbu.edu.hk; klwong@hkbu.edu.hk
bDepartment of Applied Biology and Chemical Technology, Hong Kong Polytechnic University, Hung Hum, Hong Kong SAR, China
cDepartment of Chemistry, Durham University, Durham, DH1 3LE, UK
First published on 6th December 2016
Abstract
We have synthesized a bifunctional erbium–porphyrin tumor imaging and PDT agent (Er–R3) that is capable of killing bladder cancer cells via its selective binding to the integrin αvβ3 isoform overexpressed on the cell membrane.
Photodynamic therapy (PDT) is an emerging novel cancer treatment modality suitable for repeated applications in treating diverse tumors. Since the treatment is highly localized, its systemic side-effects are relatively minimal with little adverse impact on the quality of life of the patients.1 Despite these advantages and the approval of a number of photo-sensitizers by the US Food and Drug Administration in the 1980s as a treatment option for localized (i.e., non-metastatic) cancers as well as for pre-cancerous lesions on skin and in the mouth, clinical use of PDT is still rather limited.2 This is due partly to technological constraints and partly to a lack of recognition of PDT as a medical specialty.3 Recently, with significant advances in light delivery and imaging technology, we begin to see a renaissance of PDT research and clinical translation.4–7 Nevertheless, conventional PDT drugs still suffer from several drawbacks: (i) it is only able to treat those lesions where light can penetrate, i.e., a few millimeters under the skin or from the irradiated tissue surface; (ii) some currently used PDT drugs render patients very sensitive to light and special precautions against light exposure must be taken until the drugs are cleared from the body in several days or even weeks; (iii) adverse in vitro/in vivo reactions occur due to the variation in physiological conditions and notched distribution of cytotoxic singlet oxygen; and (iv) non-specific cytotoxic activity may inflict damage to the normal cells during the PDT treatment.
Porphyrin-based compounds, the first-generation PDT drugs, have been investigated continuously since their first clinical approval in an effort to overcome these drawbacks.8–10 Recently, several porphyrin derivatives have been developed to absorb strongly in the near-infrared (NIR) region (λ > 800 nm), where the tissue-penetration depth is much higher, via multi-photon femtosecond laser excitation with pinpoint accurate targeting to reduce collateral photodamage.11,12 In addition to the precise targeting using multi-photon laser, cancer selectivity can be incorporated into the PDT agents by taking advantages of some specific characteristics of the cancer cells. One recent example is a bifunctional gadolinium–porphyrin derivative which binds specifically to the anionic membrane of the cancer cells and then exerts its PDT action after NIR-excited imaging.13
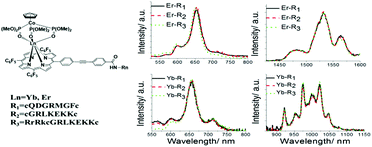
Our design of a new generation bifunctional tumor-imaging and PDT agent is based on porphyrin–lanthanide complexes, with specific functional groups which allow them to localize selectively on particular tumor types, together with responsive imaging via NIR emission from the lanthanide.14,15 In this work, the tumor selective binding of the porphyrin–erbium complexes, Er–Rn (n = 1–3), is achieved through conjugation with bladder cancer-specific as well as integrin αvβ3 isoform-specific peptides. The bladder cancer-specific peptide sequences, R1–R3 (molecular structures of R1–R3 are shown in Scheme S2, ESI†), are obtained from a combinatorial chemistry approach, with R1 further reported to be αvβ3 integrin-specific as well.16 An increased integrin αvβ3 expression has been observed in the neovasculature of bladder cancer, particularly in invasive carcinoma.16–18 Our results show that Er–R3 is able to interrupt bladder tumor growth significantly with specific lysosome localization indicated by responsive emission from Er. To increase the water solubility of the Er complexes over our previously reported analogues,13,14 a hydrophilic peptide RrRK was conjugated to the relatively more hydrophobic bladder cancer-specific peptide sequence R2 (–cGRLKEKKc–), affording the amphiphilic R3 peptide with an improved cell membrane permeability. The absorption coefficients and emission quantum yields of Er–R1, Er–R2 and Er–R3 are similar. The results of the photophysical measurement of Ln–Rn are shown in Table 1. Er exhibits stronger singlet oxygen quantum efficiency than Yb due to the fact that energy transfer from porphyrin to Yb for f–f emission is much better than that to Er for f–f emission, resulting in more excitation energy being channeled to singlet oxygen production.
| Compound | Absorptiona (λmax) [nm] log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (ε[dm3 mol−1 cm−1]) (ε[dm3 mol−1 cm−1]) |
Emissiona (λem) [nm] (Φ = τ)b,c | Φ Δ |
|---|---|---|---|
| a Absorption and emission were measured in water (3% DMSO) at room temperature. b The emission quantum yield standard used was tetraphenylporphyrin (H2TPP) in anhydrous DCM (Φem = 0.120 at 298 K). c Lifetime was measured in water (3% DMSO) at room temperature. d The singlet oxygen quantum yield measured was referenced to tetraphenylporphyrin (H2TPP) in anhydrous DCM (ΦΔ = 0.62 at 298 K). | |||
| Yb–Rl | 425(5.37), 554(4.09) | 656, 712(0.012), 975(29.86 μs) | Not found |
| Yb–R2 | 425(5.34), 554(4.16) | 656, 712(0.013), 975(30.08 μs) | Not found |
| Yb–R3 | 425(5.27), 554(4.04) | 656, 712(0.013), 975(29.97 μs) | Not found |
| Er–Rl | 426(5.32), 554(4.05) | 654, 715(0.014), 1531 | 0.11 |
| Er–R2 | 426(5.50), 554(4,53) | 654, 715(0.014), 1531 | 0.12 |
| Er–H3 | 426(5.36), 554(4.24) | 654, 715(0.015), 1531 | 0.12 |
In Fig. 1, the photophysical properties of Er or Yb porphyrin-based complexes are similar. However, the in vitro subcellular localization uptake and cytotoxicity (light and dark) are different due to the conjugated peptides. First of all, the subcellular localizations of Er–Rn and Yb–Rn complexes (n = 1, 2 and 3) in bladder cancer 5637 and T24 cells, cervical cancer HeLa cells and normal lung MRC5 cells (Fig. 2 and Fig. S24, ESI†) are different (dosed concentration = 5 μM; incubation time = 6 hours). The in vitro fluorescence intensity of the three Er complexes is higher than those of their Yb counterparts. This is due to an efficient energy transfer from the porphyrin to the Yb3+ ion which emits in the near-infrared region. In the bladder cancer 5637 and T24 cells, red porphyrin emission from Er–R1 is found only on the cell membrane, while the red emissions from Er–R2 and Er–R3 are found inside the cells. Their Yb analogues also showed the same subcellular localization pattern, i.e., the porphyrin emission from Yb–R1 is found on the cell membrane while Yb–R2 and Yb–R3 are found within the cells. Co-localization experiments using green LysoTracker were conducted. The red emissions from Er–R2, Er–R3, Yb–R2 and Yb–R3 were observed to overlap well with the green fluorescence from the LysoTracker in the 5637 and T24 cells (Fig. S22, ESI†). No such overlap was seen with Er–R1 and Yb–R1. These observations clearly indicate that the Er–R2, Er–R3, Yb–R2 and Yb–R3 complexes are mostly localized in the lysosomes of the 5637 and T24 cells. Er–R1 and Yb–R1, however, are localized in the 5637 and T24 cell membrane. This is consistent with the reported specificity of the conjugated R1 peptide towards integrin αvβ3 over-expressed on the bladder cancer cell membrane.
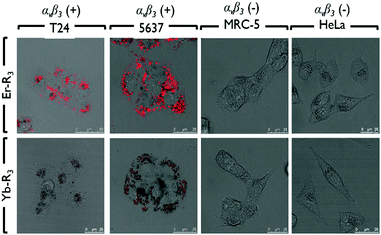 | ||
| Fig. 2 Subcellular localization of Er–R3 and Yb–R3 in human bladder carcinoma (T24 and 5637) cells, normal lung fibroblast (MRC-5) cells, and human cervical carcinoma (HeLa) cells. | ||
To confirm that the peptide sequences R1, R2 and R3 recognize bladder cancer specifically, in vitro imaging of Er–Rn and Yb–Rn (n = 1, 2 and 3) was performed using the non-bladder cancer cells, HeLa and MRC-5, under identical experimental conditions. No red emission was detected in HeLa or MRC-5 cells, thus showing very limited uptake of these porphyrin–lanthanide complexes. As the porphyrin complexes Er–Rn and Yb–Rn (n = 1, 2 and 3) will not bind to HeLa and MRC-5 cells, only the green emission from the LysoTracker is observed in the fluorescence staining experiment (Fig. S22, ESI†).
To confirm that the selective uptake of Er–Rn and Yb–Rn (n = 1, 2 and 3) complexes by bladder cancer cells was due to the recognition of the αvβ3 integrin on the 5637 and T24 cell surface, cellular uptake of these complexes functionalized with different peptides R1, R2 and R3 was studied by flow cytometry on the 5637, T24, HeLa and MRC-5 cells. Both R1 and R2 peptides were shown to exhibit specific binding towards bladder cancer through screening using the one-bead one-compound (OBOC) combinatorial peptide library technology,19 with R1 further reported to bind to the αvβ3 integrin on the T24 cell. The bladder cancer-specific binding of R1 was further demonstrated in vivo on a xenograft mouse model.20R3 is designed as an amphiphilic peptide modified from R2 by the addition of a hydrophilic peptide RrRK to its N-terminal to enhance its cell permeability. The results from the flow cytometric cell uptake experiments are shown in Fig. S23 (ESI†). From Fig. S23 (ESI†), no significant uptake of the Er–Rn and Yb–Rn (n = 1, 2 and 3) complexes is observed in HeLa and MRC-5 cells even after 24 hours of incubation. In contrast, substantial uptake of these complexes by the 5637 and T24 cells is clearly seen after 6 hours of incubation. Regarding the Er and Yb complexes functionalized with different peptides, their uptake rates by the 5637 and T24 cells showed the following trend: R3 > R2 > R1. This observation is also reflected in the median fluorescence intensity recorded for these complexes after 24 hours of incubation (Table S2, ESI†).
After verifying the specific uptake of Er–Rn and Yb–Rn complexes into T24 cells, in vitro PDT in various cell lines was carried out. A clinically approved conventional PDT agent, aminolevulinic acid (ALA), which exhibits no specific tumor selectivity, was used for comparison as well.21 An ideal PDT photosensitizer should have a low dark cytotoxicity (i.e., a high dark IC50) and a high photo-cytotoxicity (i.e., a low light IC50).
These two contrasting properties can be summarized in terms of a photodynamic therapeutic index, PTI, which is defined as the ratio of the dark IC50 over light IC50 of the PDT agent. The cytotoxicity of Er–Rn and Yb–Rn complexes towards T24, HeLa and MRC-5 cells was measured in dark and under photo-irradiation (550 nm long-pass filter, 6 mW cm−2, 28 min) using MTT assay. The results are shown in Fig. 3. These complexes exhibited high photo-cytotoxicity under a light dose of 10 J cm−2. Furthermore, their photo-cytotoxicity increased with increasing concentrations of the Er–Rn and Yb–Rn complexes. The IC50 of Er–Rn and Yb–Rn complexes to the T24 cells is 8–10 fold lower than those towards the HeLa and MRC-5 cells, thus demonstrating their selective PDT activities towards bladder cancer. Due to the amphiphilic character of the R3 peptide in Er–R3 and Yb–R3, the cellular uptake of these complexes is higher than those of Er–R1, Er–R2, Yb–R1 and Yb–R2, thus resulting in their higher photo-cytotoxicity. In comparison, the ALA-PDT activity towards the T24 cells is 4–8 fold lower than those of the Er–Rn and Yb–Rn complexes. As for the HeLa and MRC-5 cells, ALA showed photo-cytotoxicity either comparable to or lower than those of the Er–Rn and Yb–Rn complexes. Among all of the Er–Rn and Yb–Rn complexes, Er–R3 shows the highest PTI of ca. 34, followed by Er–R2 > Er–R1. A similar trend is seen with the Yb–Rn complexes: Yb–R3 > Yb–R2 > Yb–R1. The dark cytotoxicity of all these complexes is very low (with their dark IC50 of over 1000 μM). Based on these results, Er–R3 is the most promising candidate as a new generation PDT agent to selectively kill bladder cancer. Since a peptide specific for a particular cancer or stem cell type can be identified from a phage-displayed random peptide,22–24 our new generation erbium porphyrin complex can be extended not only to targeted imaging and destruction of any cancer type but also to tracking stem cell migration.
In conclusion, we present a multi-modal lanthanide–porphyrin PDT agent that is capable of killing tumor cells via1O2 produced from a porphyrin moiety, affording fluorescence imaging simultaneously. Er–R3 is synthesized, and it shows high selectivity for bladder cancer cells by specifically targeting the integrin αvβ3 isoform with strong NIR emission and 1O2 generation. The selective uptake of our complexes by cancer cells is confirmed by flow cytometry and in vitro imaging, and they are able to significantly interrupt the growth of bladder cancer cells via specific binding to the “integrin αvβ3 isoform”.
This work was supported by the Hong Kong Baptist University (HKBU) Faculty Research Grants (FRG2/14-15/013), the Hong Kong Polytechnic University (HKPolyU), the Hong Kong Research Grants Council (HKBU 22301615, Polyu 253002/14P and Polyu 5096/13P) and the HKBU-HKPolyU Joint Research Programme (RC-ICRS/15-16/02F-KLW and RC-ICRS/15-16/02D-DWJK).
Notes and references
- I. Yoon, J. Z. Li and Y. K. Shim, Clin. Endosc., 2013, 46, 7–23 CrossRef PubMed.
- D. E. J. G. J. Dolmans, D. Fukumura and R. K. Jain, Nat. Rev. Cancer, 2003, 3, 380–387 CrossRef CAS PubMed.
- K. Moghissi, Photodiagn. Photodyn. Ther., 2011, 8, 73–74 CrossRef PubMed.
- J. Chen, L. Keltner, J. Christophersen, F. Zheng, M. Krouse, A. Singhal and S.-S. Wang, Cancer J., 2002, 8, 154–163 CrossRef PubMed.
- S. Mordon, C. Cochrane, J. B. Tylcz, N. Betrouni, L. Montier and V. Koncar, Photodiagn. Photodyn. Ther., 2015, 12, 1–8 CrossRef CAS PubMed.
- T. Nakamura, T. Oinuma, H. Yamagishi, H. Masuyama and A. Terano, Photodiagn. Photodyn. Ther., 2015, 12, 115–122 CrossRef PubMed.
- H. Lee, Y. Lee, C. Song, H. R. Cho, R. Ghaffari, T. K. Choi, K. H. Kim, Y. B. Lee, D. Ling, H. Lee, S. J. Yu, S. H. Choi, T. Hyeon and D.-H. Kim, Nat. Commun., 2015, 6, 10059 CrossRef CAS PubMed.
- L. G. Arnaut, Adv. Inorg. Chem., 2011, 63, 187–233 CrossRef CAS.
- M. Ethirajan, Y. Chen, P. Joshi and R. K. Pandey, Chem. Soc. Rev., 2011, 40, 340–362 RSC.
- V. Bogoeva, M. Siksjo, K. G. Saeterbo, T. B. Melo, A. Bjorkoy, M. Lindgren and O. A. Gederaas, Photodiagn. Photodyn. Ther., 2016, 14, 9–17 CrossRef CAS PubMed.
- E. Dahlstedt, H. A. Collins, M. Balaz, M. K. Kuimova, M. Khurana, B. C. Wilson, D. Phillips and H. L. Anderson, Org. Biomol. Chem., 2009, 7, 897–904 CAS.
- C.-T. Poon, P.-S. Chan, C. Man, F.-L. Jiang, R. N. S. Wong, N.-K. Mak, D. W. J. Kwong, S.-W. Tsao and W.-K. Wong, J. Inorg. Biochem., 2010, 104, 62–70 CrossRef CAS PubMed.
- T. Zhang, R. Lan, C.-F. Chan, G.-L. Law, W.-K. Wong and K.-L. Wong, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, E5492–E5497 CrossRef CAS PubMed.
- T. Zhang, C.-F. Chan, J. Hao, G.-L. Law, W.-K. Wong and K.-L. Wong, RSC Adv., 2013, 3, 382–385 RSC.
- G. L. Law, R. Pal, L. O. Palsson, D. Parker and K.-L. Wong, Chem. Commun., 2009, 7321–7323 RSC.
- M. D. Sachs, K. A. Rauen, M. Ramamurthy, J. L. Dodson, A. M. De Marzo, M. J. Putzi, M. P. Schoenberg and R. Rodriguez, Urology, 2002, 60, 531–536 CrossRef PubMed.
- S. Liu, S. P. Robinson and D. S. Edwards, Top. Curr. Chem., 2005, 252, 193–216 CAS.
- T. Saito, M. Kimura, T. Kawasaki, S. Sato and Y. Tomita, Br. J. Cancer, 1996, 73, 327–331 CrossRef CAS PubMed.
- H. Zhang, O. H. Aina, K. S. Lam, R. de Vere White, C. Evans, P. Henderson, P. N. Lara, X. Wang, J. A. Bassuk and C. X. Pan, Urol. Oncol., 2012, 30, 635–645 CrossRef CAS PubMed.
- T. Y. Lin, H. Zhang, S. Wang, L. Xie, B. Li, C. O. Rodriguez, R. de Vere White and C. X. Pan, Mol. Cancer, 2011, 10, 9 CrossRef PubMed.
- M.-C. Tetard, M. Vermandel, S. Mordon, J.-P. Lejeune and N. Reyns, Photodiagn. Photodyn. Ther., 2014, 11, 319–330 CrossRef CAS PubMed.
- G. Abbineni, S. Modali and C. Mao, Mol. Pharmaceutics, 2010, 7, 1629–1642 CrossRef CAS PubMed.
- N. Gandra, G. Abbineni and C. Mao, Small, 2013, 9, 215–221 CrossRef CAS PubMed.
- K. Ma, D. Wang, Y. Lin and C. Mao, Adv. Funct. Mater., 2013, 23, 1172–1181 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cc09246b |
| This journal is © The Royal Society of Chemistry 2017 |