Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A platinum(II)–palladium(II)–nickel(II) heterotrimetallic coordination polymer showing a cooperative effect on catalytic hydrogen evolution†
Naoto
Kuwamura
,
Yoshinari
Kurioka
and
Takumi
Konno
 *
*
Department of Chemistry, Graduate School of Science, Osaka University, Toyonaka, Osaka 560-0043, Japan. E-mail: konno@chem.sci.osaka-u.ac.jp
First published on 5th December 2016
Abstract
Stepwise construction of a 1D heterotrimetallic coordination polymer containing all three group 10 metal ions via an ammineplatinum(II) metalloligand with D-penicillamine is reported. This system showed a significant enhancement in heterogeneous catalytic activity for electrochemical hydrogen evolution by the stepwise introduction of PdII and NiII into the PtII metalloligand.
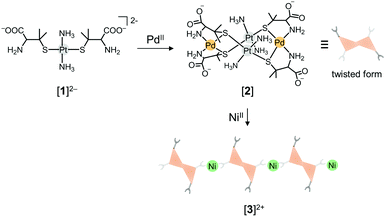
In recent years, heterometallic coordination compounds have received increased attention because the incorporation of different kinds of metal ions into one molecule often leads to an intriguing cooperative effect on physical and chemical properties, such as magnetism, emission, and catalysis.1 To date, many examples of ‘heterobimetallic’ compounds that show some cooperative effect on their properties have been reported. However, reports on a cooperative effect for ‘heterotrimetallic’ species are still relatively uncommon.2 This is owing to a lack of rational synthetic methods to incorporate three different kinds of metal ions into a single molecule; most of the heterotrimetallic coordination compounds have been synthesized via one-pot reactions in which three kinds of metal ions are self-assembled with organic and/or inorganic ligands under specific conditions.3 To overcome this problem, we have developed a metalloligand approach, in which a metal complex having several donor sites coordinates in a stepwise fashion to second and third metal ions to form the desired heterotrimetallic coordination compounds.4–6 Previously, we showed that a linear-type AuI complex, [Au(D-pen)2]3− (D-H2pen = D-penicillamine), in which an AuI centre is linearly coordinated by two thiolato groups from two D-pen ligands, can serve as a multifunctional metalloligand by using coordinated thiolato and free amine and carboxylate groups, forming a variety of heterobimetallic and heterotrimetallic species.6 For example, a cage-type metallosupramolecular compound that contains all group 11 metal ions (AuI, AgI, and CuII) has been synthesized by the stepwise reaction of [Au(D-pen)2]3− with Ag+ and Cu2+.6a This was the first example of a heterotrimetallic coordination compound consisting of all group 11 metal ions. Other heterotrimetallic compounds that consist of all metal ions belonging to the same group have rarely been synthesized.7 Recently, we found that a newly prepared PtII metalloligand with D-pen, trans-[Pt(NH3)2(D-pen)2]2− ([1]2−), in which the AuI linker in [Au(D-pen)2]3− is replaced by a trans-{PtII(NH3)2}2+ moiety, also acts as a multifunctional metalloligand.8 This finding prompted us to investigate the reactivity of [1]2− toward Pd2+ and Ni2+ to create a heterotrimetallic compound containing all group 10 metal ions (PtII, PdII, and NiII) in one molecule. Here, we report that [1]2− reacts with Pd(OAc)2 to afford a PtII2PdII2 tetranuclear complex ([2]), which further reacts with NiCl2 to produce a PtII2PdII2NiII coordination polymer ([3]Cl2) (Scheme 1). Remarkably, [3]Cl2 showed excellent catalytic activity for electrochemical hydrogen evolution compared with [1]2− and [2], indicating a significant enhancement of the catalytic activity due to the incorporation of all three group 10 metal ions in one molecule. To our knowledge, such a cooperative effect on catalytic activity has not been reported.
The reaction of [Pt(NH3)2(D-Hpen)2] ([H21]) with Pd(OAc)2 in H2O gave an orange solution, from which yellow crystals ([2]) were isolated. The 1H NMR spectrum of [2] displayed only a single set of signals (δ 1.43, 1.56, and 3.35 ppm) due to D-pen ligands in the complex, indicative of its high symmetrical structure (Fig. S1, ESI†). The presence of a deprotonated carboxyl group in [2] was implied by its IR spectrum that shows a strong band at 1600 cm−1 (Fig. S2, ESI†).9 The elemental analytical data of [2] were in agreement with a neutral formula containing [1]2− and PdII in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
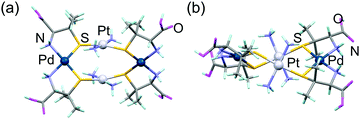
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Single crystals of [2] suitable for X-ray crystallography were obtained as the protonated form, [H42]Cl4. The asymmetric unit of [H42]Cl4 contains one complex cation and four chloride anions, besides solvated water molecules. As shown in Fig. 1, the complex cation has an S-bridged PtII2PdII2 tetranuclear structure in [Pd2Pt2(NH3)4(D-Hpen)4]4+, consisting of two metalloligands with protonated carboxyl groups ([H21]) and two PdII atoms. Each of the two metalloligands span two PdII atoms in a bis(bidentate-N,S) mode, such that each PdII centre has a square planar geometry with a cis-N2S2 donor set (av. Pd–N: 2.084 Å, Pd–S: 2.272 Å). The Pt⋯Pt distance in [H42]4+ is 3.300 Å, indicating the lack of an intramolecular Pt⋯Pt interaction.10 Each NH3 ligand is hydrogen-bonded with a D-pen thiolato group (av. NNH3⋯S = 3.46 Å), which appears to stabilize the PtII2PdII2 structure in [2]. Here, it should be noted that the 1H NMR spectral features of the reaction solution are the same as those of isolated [2] (Fig. S1, ESI†), indicating that only a single isomer with two cis-[PdN2S2] planes is selectively formed by the reaction of [H21] with Pd2+. This result is different from the corresponding reaction of [Au(D-pen)2]3− with Pd2+, which produced a mixture of cis and trans isomers of [Au2Pd2(D-pen)4]2−.6c The overall structure of [H42]4+ resembles that of the cis isomer of [Au2Pd2(D-pen)4]2−. However, all of the bridging S atoms in [H42]4+ have the R configuration and form a twisted PtII2PdII2S4 framework with a D2 molecular symmetry (Fig. 1). In contrast, cis-[Au2Pd2(D-pen)4]2− has two S and two R configurational bridging S atoms, forming a boat-like framework with a C2 symmetry.6c While a number of S-bridged M2M2′ tetranuclear complexes with two MN2S2 square-planes have been prepared and structurally characterized,11 the formation of the twisted form is rare. Molecular model examinations revealed that, in [H42]4+, there exist steric repulsions between the NH3 ligand and the methyl group of a D-pen ligand in the trans isomer and the boat and chair forms of the cis isomer (Fig. S3, ESI†).
1 ratio. Single crystals of [2] suitable for X-ray crystallography were obtained as the protonated form, [H42]Cl4. The asymmetric unit of [H42]Cl4 contains one complex cation and four chloride anions, besides solvated water molecules. As shown in Fig. 1, the complex cation has an S-bridged PtII2PdII2 tetranuclear structure in [Pd2Pt2(NH3)4(D-Hpen)4]4+, consisting of two metalloligands with protonated carboxyl groups ([H21]) and two PdII atoms. Each of the two metalloligands span two PdII atoms in a bis(bidentate-N,S) mode, such that each PdII centre has a square planar geometry with a cis-N2S2 donor set (av. Pd–N: 2.084 Å, Pd–S: 2.272 Å). The Pt⋯Pt distance in [H42]4+ is 3.300 Å, indicating the lack of an intramolecular Pt⋯Pt interaction.10 Each NH3 ligand is hydrogen-bonded with a D-pen thiolato group (av. NNH3⋯S = 3.46 Å), which appears to stabilize the PtII2PdII2 structure in [2]. Here, it should be noted that the 1H NMR spectral features of the reaction solution are the same as those of isolated [2] (Fig. S1, ESI†), indicating that only a single isomer with two cis-[PdN2S2] planes is selectively formed by the reaction of [H21] with Pd2+. This result is different from the corresponding reaction of [Au(D-pen)2]3− with Pd2+, which produced a mixture of cis and trans isomers of [Au2Pd2(D-pen)4]2−.6c The overall structure of [H42]4+ resembles that of the cis isomer of [Au2Pd2(D-pen)4]2−. However, all of the bridging S atoms in [H42]4+ have the R configuration and form a twisted PtII2PdII2S4 framework with a D2 molecular symmetry (Fig. 1). In contrast, cis-[Au2Pd2(D-pen)4]2− has two S and two R configurational bridging S atoms, forming a boat-like framework with a C2 symmetry.6c While a number of S-bridged M2M2′ tetranuclear complexes with two MN2S2 square-planes have been prepared and structurally characterized,11 the formation of the twisted form is rare. Molecular model examinations revealed that, in [H42]4+, there exist steric repulsions between the NH3 ligand and the methyl group of a D-pen ligand in the trans isomer and the boat and chair forms of the cis isomer (Fig. S3, ESI†).
To create a heterotrimetallic coordination compound containing all group 10 metal ions (PtII, PdII, and NiII), [2] was reacted with NiCl2 in water. This reaction gave a clear green solution, from which yellow crystals of [3]Cl2 were obtained. X-ray fluorescence analyses indicated the presence of Pt, Pd, and Ni atoms in [3]Cl2, and its elemental analytical data were consistent with a formula containing [2] and NiCl2 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
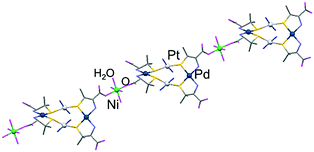
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The IR spectrum of [3]Cl2 showed a strong absorption band at 1640 cm−1, indicating the presence of deprotonated carboxylate groups (Fig. S2, ESI†).9 The structure of [3]Cl2 was established by a single-crystal X-ray diffraction study. The asymmetric unit of [3]Cl2 contains one [Pd2Pt2(NH3)4(D-pen)4] molecule, one NiII ion, and two chloride anions, besides solvated water molecules. As shown in Fig. 2, the overall structure of the PtII2PdII2 tetranuclear molecule in [3]Cl2 is essentially the same as that of [H42]Cl4. However, the four carboxyl groups of the PtII2PdII2 molecule in [3]Cl2 are deprotonated, consistent with the IR spectral feature. In addition, two of these groups each coordinate to a NiII ion to form a cationic 1D chain structure in [{Ni(H2O)4}{Pd2Pt2(NH3)4(D-pen)4}]2+ (Fig. 2). Note that [3]Cl2 is the first example of a structurally characterized coordination compound with all three group 10 metal ions. In [3]2+, each NiII ion is bound by four aqua ligands and two carboxylate groups in a trans octahedral geometry (av. Ni–OCOO−: 2.064 Å, Ni–Owater: 2.093 Å). The chloride ions are not involved in the coordination and are located between the PtII2PdII2NiII chains, forming hydrogen bonds with ammine groups and water molecules (av. Cl⋯NNH3: 3.29 Å, Cl⋯Owater: 3.09 Å, Fig. S3, ESI†).
1 ratio. The IR spectrum of [3]Cl2 showed a strong absorption band at 1640 cm−1, indicating the presence of deprotonated carboxylate groups (Fig. S2, ESI†).9 The structure of [3]Cl2 was established by a single-crystal X-ray diffraction study. The asymmetric unit of [3]Cl2 contains one [Pd2Pt2(NH3)4(D-pen)4] molecule, one NiII ion, and two chloride anions, besides solvated water molecules. As shown in Fig. 2, the overall structure of the PtII2PdII2 tetranuclear molecule in [3]Cl2 is essentially the same as that of [H42]Cl4. However, the four carboxyl groups of the PtII2PdII2 molecule in [3]Cl2 are deprotonated, consistent with the IR spectral feature. In addition, two of these groups each coordinate to a NiII ion to form a cationic 1D chain structure in [{Ni(H2O)4}{Pd2Pt2(NH3)4(D-pen)4}]2+ (Fig. 2). Note that [3]Cl2 is the first example of a structurally characterized coordination compound with all three group 10 metal ions. In [3]2+, each NiII ion is bound by four aqua ligands and two carboxylate groups in a trans octahedral geometry (av. Ni–OCOO−: 2.064 Å, Ni–Owater: 2.093 Å). The chloride ions are not involved in the coordination and are located between the PtII2PdII2NiII chains, forming hydrogen bonds with ammine groups and water molecules (av. Cl⋯NNH3: 3.29 Å, Cl⋯Owater: 3.09 Å, Fig. S3, ESI†).
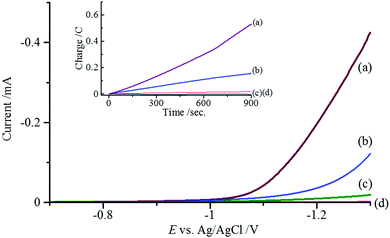
The catalytic property of [3]Cl2 in the solid state was electrochemically investigated, and the results were compared with those of [H21] and [2]. The linear sweep voltammetry of a solid sample of [3]Cl2, attached to the surface of a glassy carbon electrode (0.07 cm2), in H2O/CH3CN (v/v = 1/9) containing 0.1 M LiClO4 displayed a sharp current increase at an onset potential of −0.98 V (vs. Ag/AgCl). A similar but lower current increase was also observed for a solid sample of [2] at −1.10 V. (Fig. 3).12 However, a solid sample of [H21] showed only a slight current increase at −1.15 V. Since the control experiment using a bare glassy carbon electrode under the same conditions did not show any current increase until reaching −1.5 V, this observation is indicative of a catalytic reduction,13 the activity of which drastically increases in the following order: [H21] < [2] < [3]Cl2. During the voltammetric scans, the evolution of bubbles from the electrode surface was observed for [2] and [3]Cl2. The bubbles were analyzed using gas chromatography (GC) and found to be H2 gas (Fig. S5, ESI†). Such an evolution of bubbles, as well as a current increase, was not detected when absolute CH3CN was used as the solvent instead of H2O/CH3CN (Fig. S6, ESI†). Thus, [2] and [3]Cl2 catalyze the electrochemical reduction of water to produce H2 gas. These are relatively few examples of coordination compounds that exhibit a heterogeneous catalytic activity for H2 gas evolution on an electrode surface.14 Considering that [H21] is significantly less active for catalytic reduction of water than [2] and [3]Cl2, the catalytic reaction mainly occurs at the PdII centre of the [Pd(D-pen)2]2− square-planes. It has been proposed that Pd is the best alternative to Pt in catalytic systems for H2 evolution.15 However, examples reporting catalytic hydrogen evolution are limited to several homogeneous catalytic systems,16 and this is the first PdII coordination system that evidences heterogeneous catalytic H2 evolution.
To evaluate the H2 evolution rate, potential-controlled electrolyses were carried out under the same conditions as those of the voltammetric experiments. At an applied potential of −1.20 V, the rate of Coulomb charge increased in the order [H21] < [2] < [3]Cl2, which is in parallel with the voltammetric results (Fig. 3). The turnover frequency (TOF) of H2 (mol H2/mol cat) was calculated to be 3.73, 18.2, and 34.4 mol min−1 cm−2 for [H21], [2], and [3]Cl2, respectively. The GC analysis indicated that the amount of H2 gas generated during the electrolysis using [2] and [3]Cl2 was 1.56 μmol and 2.88 μmol, respectively. From these values, the Faraday efficiencies for [2] and [3]Cl2 were calculated to be 81% and 100%, respectively, indicating that no side redox reaction occurs during the electrolysis for [3]Cl2. It has been shown that the increase of Lewis acidity around the reaction centre promotes the efficiency of an electrocatalytic hydrogen evolution.17 In [3]Cl2, the D-pen carboxylate group coordinates to a NiII centre, which might lead to an increase of the Lewis acidity around a PdII catalytic centre.18
In summary, we showed that the metalloligand trans-[Pt(NH3)2(D-pen)2]2− ([1]2−) reacts with PdII to afford an S-bridged PtII2PdII2 complex, [Pd2Pt2(NH3)4(D-pen)4] ([2]), which exclusively forms a twisted isomer consisting of cis-[Pd(D-pen)2]2− planes. This coordination behaviour is due to the presence of two NH3 ligands bound to each PtII centre, exerting non-bonding steric and NH⋯S hydrogen bonding interactions. Complex [2] acts as an O-donating metalloligand to NiII to create a PtII2PdII2NiII coordination polymer, [{Ni(H2O)4}{Pd2Pt2(NH3)4(D-pen)4}]Cl2 ([3]Cl2), which is the first example of a structurally characterized heterotrimetallic coordination compound with all three group 10 metal ions in one molecule. Due to the stepwise combination of PdII and NiII centres with [1]2−, the electrocatalytic water reduction was largely enhanced by heterogeneous activity, demonstrating a significant cooperative effect due to metal ions belonging to the same group, for the first time.
This work was supported by CREST, JST and JSPS KAKENHI Grant Numbers 16K13609 and 16K17945. One of the authors (NK) acknowledges the support from the Kurita Water and Environment Foundation and the Kansai Research Foundation for Technology Promotion.
Notes and references
-
(a) S. L.-F. Chan, S. Gao, S. S.-Y. Chui, L. Shek, J.-S. Huang and C.-M. Che, Chem. – Eur. J., 2012, 18, 11228–11237 CrossRef CAS PubMed
; (b) R. Matsuda, Bull. Chem. Soc. Jpn., 2013, 86, 1117–1131 CrossRef CAS
; (c) P. Amo-Ochoa and F. Zamora, Coord. Chem. Rev., 2014, 276, 34–58 CrossRef CAS
; (d) Y. Hasegawa, Bull. Chem. Soc. Jpn., 2014, 87, 1029–1057 CrossRef CAS
; (e) X.-L. Yang and C.-D. Wu, CrystEngComm, 2014, 16, 4907–4918 RSC
; (f) J.-W. Zhang, X.-M. Kan, X.-L. Li, J. Luan and X.-L. Wang, CrystEngComm, 2015, 17, 3887–3907 RSC
; (g) D. Bansal, S. Pandey, G. Hundal and R. Gupta, New J. Chem., 2015, 39, 9772–9781 RSC
.
-
(a) A. Singh and R. C. Mehrotra, Coord. Chem. Rev., 2004, 248, 101–118 CrossRef CAS
; (b) L. John and P. Sobata, Acc. Chem. Res., 2014, 47, 470–481 CrossRef CAS PubMed
; (c) T. R. Cook and P. J. Stang, Chem. Rev., 2015, 115, 7001–7045 CrossRef CAS PubMed
; (d) H. Li, Z.-J. Yao and G.-X. Jin, Coord. Chem. Rev., 2015, 293–294, 139–157 CrossRef CAS
.
-
(a)
R. E. P. Winpenny, Comprehensive Coordination Chemistry II, Elsevier Pergamon, Oxford, 2004 Search PubMed
; (b) E. K. Brechin, Chem. Commun., 2005, 5141–5153 RSC
; (c) D. S. Nesterov, V. N. Kokozay and B. W. Skelton, Eur. J. Inorg. Chem., 2009, 5469–5473 CrossRef CAS
.
-
(a) T. Konno, Bull. Chem. Soc. Jpn., 2004, 77, 627–649 CrossRef CAS
; (b) A. Igashira-Kamiyama and T. Konno, Dalton Trans., 2011, 40, 7249–7263 RSC
; (c) N. Yoshinari and T. Konno, Chem. Rec., 2016, 16, 1647–1663 CrossRef CAS PubMed
.
-
(a) T. Konno, Y. Chikamoto, K. Okamoto, T. Yamaguchi, T. Ito and M. Hirotsu, Angew. Chem., Int. Ed., 2000, 39, 4098–4101 CrossRef CAS
; (b) T. Aridomi, M. Hirotsu, T. Yoshimura, T. Kawamoto and T. Konno, Chem. Lett., 2005, 34, 770–771 CrossRef CAS
; (c) Y. Chikamoto, T. Kawamoto, A. Igashira-Kamiyama and T. Konno, Inorg. Chem., 2005, 44, 1601–1610 CrossRef CAS PubMed
; (d) N. Yoshinari, Y. Chikamoto, M. Iwata, T. Kawamoto and T. Konno, Bull. Chem. Soc. Jpn., 2006, 79, 1745–1747 CrossRef
; (e) T. Aridomi, K. Takamura, A. Igashira-Kamiyama and T. Konno, Chem. Lett., 2008, 37, 170–171 CrossRef CAS
; (f) T. Aridomi, A. Igashira-Kamiyama and T. Konno, Inorg. Chem., 2008, 14, 7752–7755 CAS
; (g) N. Yoshinari, A. Igashira-Kamiyama and T. Konno, Chem. – Eur. J., 2010, 16, 14247–14251 CrossRef CAS PubMed
; (h) N. Yoshinari and T. Konno, Dalton Trans., 2011, 40, 12191–12200 RSC
; (i) K. Igawa, N. Yoshinari and T. Konno, Chem. Commun., 2014, 50, 15573–15576 RSC
.
-
(a) A. Toyota, T. Yamaguchi, A. Igashira-Kamiyama, T. Kawamoto and T. Konno, Angew. Chem., Int. Ed., 2005, 44, 1088–1092 CrossRef CAS PubMed
; (b) M. Taguchi, A. Igashira-Kamiyama, T. Kajiwara and T. Konno, Angew. Chem., Int. Ed., 2007, 46, 2422–2425 CrossRef CAS PubMed
; (c) M. Taguchi, Y. Sameshima, A. Igashira-Kamiyama, S. Akine, T. Nabeshima and T. Konno, Chem. Lett., 2008, 37, 244–245 CrossRef CAS
; (d) T. Konno, N. Yoshinari, M. Taguchi and A. Igashira-Kamiyama, Chem. Lett., 2009, 38, 526–527 CrossRef CAS
; (e) T. Konno, A. Toyota and A. Igashira-Kamiyama, J. Chin. Chem. Soc., 2009, 38, 26–33 CrossRef
; (f) S. Surinwong, N. Yoshinari, B. Yotnoi and T. Konno, Chem. – Asian J., 2016, 11, 486–490 CrossRef CAS PubMed
; (g) S. Surinwong, N. Yoshinari, T. Kojima and T. Konno, Chem. Commun., 2016, 52, 12893–12896 RSC
.
- Tanase et al. reported an AuIAgICuI octanuclear complex by the reaction of [AuI2Cl(tetraphosphine)2]Cl with AgI and CuI: Y. Takemura, T. Nishida, B. Kure, T. Nakajima and T. Tanase, Chem. – Eur. J., 2011, 17, 10528–10532 CrossRef CAS PubMed
.
- Y. Kurioka, N. Kuwamura, N. Yoshinari, A. Igashira-Kamiyama and T. Konno, Chem. Lett., 2015, 44, 1330–1332 CrossRef CAS
.
-
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Wiley, Chichester, 5th edn, 1997 Search PubMed
.
- B.-C. Tzeng, W.-F. Fu, C.-M. Che, H.-Y. Chao, K.-K. Cheung and S.-M. Peng, J. Chem. Soc., Dalton Trans., 1999, 1017–1024 RSC
.
-
(a) E. Bouwman and J. Reedijk, Coord. Chem. Rev., 2005, 249, 1555–1581 CrossRef CAS
; (b) Y. Ohki and K. Tatsumi, Eur. J. Inorg. Chem., 2011, 973–985 CrossRef CAS
; (c) J. A. Denny and M. Y. Darensbourg, Chem. Rev., 2015, 115, 5248–5273 CrossRef CAS PubMed
.
- The overpotentials at 10 mA cm−2 were 0.79 V and 0.69 V for [2] and [3]Cl2, respectively.
-
(a) P. Zhang, M. Wang, Y. Yang, D. Zheng, K. Han and L. Sun, Chem. Commun., 2014, 50, 14153–14156 RSC
; (b) D. J. Martin, B. D. McCarthy, C. L. Doniey and J. L. Dempsey, Chem. Commun., 2015, 51, 5290–5293 RSC
; (c) X. Liu, S. Cui, Z. Sun and P. Du, Chem. Commun., 2015, 51, 12954–12957 RSC
; (d) B. B. Beyene, S. B. Mane and C.-H. Hung, Chem. Commun., 2015, 51, 15067–15070 RSC
.
-
(a) O. Tantani, E. Anxolabéhère-Mallart, A. Aukauloo and P. Millet, Electrochem. Commun., 2007, 9, 54–58 CrossRef
; (b) X. L. Gao, Y. Gong, P. Zhang, Y. X. Yang, J. P. Meng, M. M. Zhang, J. L. Yin and J. H. Lin, CrystEngComm, 2014, 16, 8492–8499 RSC
; (c) A. J. Clough, J. W. Yoo, M. H. Mecklenburg and S. C. Marinescu, J. Am. Chem. Soc., 2015, 137, 118–121 CrossRef CAS PubMed
; (d) T.-W. Chiou, T.-T. Lu, Y.-H. Wu, Y.-J. Yu, L.-K. Chu and W.-F. Liaw, Angew. Chem., Int. Ed., 2015, 54, 14824–14829 CrossRef CAS PubMed
.
-
(a) A. Chen and C. Ostrom, Chem. Rev., 2015, 115, 11999–12044 CrossRef CAS PubMed
; (b) J. Chen, G. Xia, P. Jiang, Y. Yand, R. Li, R. Shi, J. Su and Q. Chen, ACS Appl. Mater. Interfaces, 2016, 8, 13378–13383 CrossRef CAS PubMed
.
-
(a) D. Meilleur, D. Rivard, P. D. Harvey, I. Gauthron, D. Lucas and Y. Mugnier, Inorg. Chem., 2000, 39, 2909–2914 CrossRef CAS PubMed
; (b) N. W. Waggoner, L. S. Spreer, B. J. Boro, D. L. Dubois and M. L. Helm, Inorg. Chim. Acta, 2012, 380, 14–21 CrossRef CAS
; (c) J. Chu, X.-H. Xie, S.-R. Yang and S.-Z. Zhan, Inorg. Chim. Acta, 2014, 410, 191–194 CrossRef CAS
; (d) D. Sirbu, T. Turta, E. A. Gibson and C. Benniston, Dalton Trans., 2015, 44, 14646–14655 RSC
.
- H. Shao, S. K. Muduli, P. D. Tran and H. S. Soo, Chem. Commun., 2016, 52, 2948–2951 RSC
.
- A solid sample of Ni(CH3COO)24H2O, which has essentially the same octahedral trans-[Ni(RCOO)2(H2O)4] structure as that in [3]Cl2, does not show any catalytic activity for electrochemical hydrogen evolution under the same conditions (Fig. S7, ESI†). This result seems to exclude the hypothesis that the NiII center in [3]Cl2 also acts as an active site for the electrochemical hydrogen evolution.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, NMR, IR, LSV, GC, and crystal packing. CCDC 1514220 and 1514221. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc08789b |
| This journal is © The Royal Society of Chemistry 2017 |