Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A helical naphthopyran dopant for photoresponsive cholesteric liquid crystals†
Yuna
Kim
a,
Michel
Frigoli
b,
Nicolas
Vanthuyne
c and
Nobuyuki
Tamaoki
*a
aResearch Institute for Electronic Science, Hokkaido University, Kita20, Nishi10, Kita-ku, Sapporo, Hokkaido 001-0020, Japan. E-mail: tamaoki@es.hokudai.ac.jp
bUMR CNRS 8180, UVSQ, Institut Lavoisier de Versailles, Université Paris-Saclay, Versailles, France
cAix Marseille Univ, CNRS, Centrale Marseille, iSm2, Marseille, France
First published on 25th November 2016
Abstract
The first photoresponsive cholesteric liquid crystal comprising a photoisomerizable helical naphthopyran derivative dopant and a nematic liquid crystal is reported. An unprecedented helical twisting power switching ratio of over 90% allowed us to demonstrate multi-cycle rotational motion of micro-objects by UV light irradiation.
Photochromic compounds have been widely applied to supramolecular systems in recent years, acting as phototriggers that undergo photoisomerization which induce dynamic macroscopic reorganization of superstructures by light irradiation.1–3 In the case of liquid crystals, a chiral photochromophore is dissolved into an achiral nematic liquid crystal (NLC), and its molecular chirality can be transferred to the NLC medium resulting in a photo-responsive chiral nematic (N* or cholesteric) liquid crystal phase.2–6 Those photo-responsive cholesteric liquid crystals (CLCs) have been extensively utilized for light-driven polarizers, reflectors, displays, tunable solid state lasers, and molecular motor applications.6–14
Among the dopants for CLCs, helicene-like molecules are well known to provide amplified chirality transfer to the nematic host because of their intrinsic helical conformation.15,16 However, photoisomerizable helicene derivatives are rather unexplored as photoresponsive dopants compared to photochemically reversible P-type molecules, such as diarylethenes and azobenzenes.1–6
Recently, Frigoli et al. reported on the novel photochromism of helical naphthopyrans overcoming fast thermal relaxation,17,18 and we anticipated that its reversible conformational change could provide efficient superstructural manipulation of CLCs. There has been a report on a non-chiral naphthopyran derivative which induces liquid crystallinity in the nematic LC host by photoisomerization,19 whereas, to the best of our knowledge, the formation of a cholesteric superstructure and its manipulation have never been exploited based on the photoswitching of naphthopyran derivatives introduced as chiral dopants.
We describe here the first example of a naphthopyran derivative which can be a promising candidate for photocontrol of macroscopic helical superstructures exhibiting a reversible photoisomerization process accompanying a large conformational change: unprecedented large photoswitching of helical twisting power of over 90% and the most efficient induction of macroscopic rotational motion of micro-sized objects reported so far (a rotation angle of 1150° at 0.2 wt%) to the best of our knowledge, on the surface of cholesteric LC films.
Synthesis of a helical naphthopyran derivative, CHR-Hexyl (10-hexyl-3,3-diphenyl-[3H]-benzo[5,6]phenanthro[4,3-f]chromene), is described elsewhere,18 and its molecular structure and the photoisomerization process are described in Fig. 1. Photoisomerization was conducted using 365 nm (9.1 mW cm−2) and 510 nm (26.5 mW cm−2) LED light sources for both solutions and CLCs containing CHR-Hexyl. Upon UV irradiation, the closed ring form (CF) gives open metastable photoproduct(s) with the structure of o-quinone allides, which are generally coloured, owing to improved electronic delocalisation compared with the closed form. The transoid-trans (TT) isomer is more thermodynamically stable than the transoid-cis (TC) one and is the major photoproduct (up to 92% conversion ratio from CF to TT) at the photostationary state upon continuous irradiation.18 Coloured forms can reconvert to the starting material both thermally and upon visible irradiation. Detailed photochemical characteristics of CHR-Hexyl are described in ref. 18.
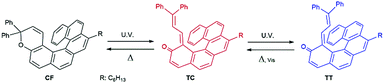 | ||
| Fig. 1 Reversible photoisomerization of CHR-Hexyl: naphthopyran (CF), transoid-cis (TC) and transoid-trans (TT). | ||
The separation of the enantiomers was performed by chiral HPLC using a semi-preparative (S,S)-Whelk-O1 column (Fig. S1, ESI†). The analytical resolution and evaluation of chiroptical properties were performed by chiral HPLC using a Chiralpak IA column (see Fig. S2a–g, ESI†) and circular dichroism (CD) spectroscopy. The two enantiomers M and P of CHR-Hexyl have different retention times (Rt) of 16.8 and 16.0 min, respectively, using dichloromethane and hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as eluent. Upon 365 nm light irradiation, the appearance of new peaks (Rt = 22.5 and 31.0 min) is assignable to the TT forms of enantiomers M and P, respectively. Subsequent thermal relaxation resulted in the recovery of the original peaks of closed ring forms of two enantiomers, and we could confirm almost no trace of racemization both after the ring opening photoisomerization and the subsequent thermal relaxation back to CF.
2) as eluent. Upon 365 nm light irradiation, the appearance of new peaks (Rt = 22.5 and 31.0 min) is assignable to the TT forms of enantiomers M and P, respectively. Subsequent thermal relaxation resulted in the recovery of the original peaks of closed ring forms of two enantiomers, and we could confirm almost no trace of racemization both after the ring opening photoisomerization and the subsequent thermal relaxation back to CF.
Chiroptical properties of the two enantiomers of CHR-Hexyl were investigated using circular dichroism (CD) spectroscopic analyses at room temperature. Fig. 2 shows CD spectra recorded for the enantiomers M and P in toluene (2.5 × 10−5 M). They show mirror image features before and after photoirradiation. The CD spectrum of M shows positive bands at 286 nm, 302 nm and 420 nm, and a strong negative band at 333 nm before UV irradiation. Upon 365 nm LED light source irradiation for the initial 30 seconds, it exhibited significant spectral change. The positive bands at 286 nm and 302 nm merged with the positive band at 299 nm with increased intensity which is consistent with the UV-Vis absorption spectral change,18 and the negative band at 333 nm shifted to 362 nm with two shoulder bands at 343 and 375 nm with decreased intensity. The small band at 420 nm disappeared and one negative and one positive band in the visible region at 452 nm and 515 nm, respectively, emerged reflecting the presence of colored forms.
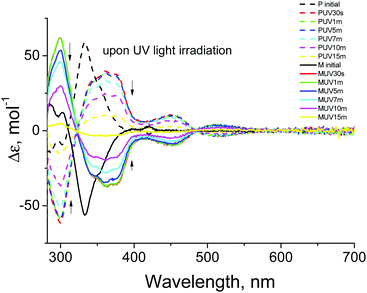 | ||
| Fig. 2 CD spectra of the toluene solution (2.5 × 10−5 M) of two enantiomers M (solid line) and P (dashed line) upon UV light irradiation. | ||
Comparing the spectra between 30 seconds and 1 minute UV irradiation, no distinctive change was observed, while subsequent UV light irradiation for more than 5 minutes resulted in continuous decreasing of the bands’ intensities. Enantiomer P also showed consistent phenomena which shows mirror features with respect to enantiomer M. The clear difference between 0 and 30 second irradiation followed by the subtle transition to 1 minute is attributable to the transition from CF to TC. Consecutive irradiation resulted in TT formation leading to a reduction of its helicity which was apparently observed from the decreasing of the band intensities. After 15 minutes of UV irradiation, thermal back reaction from TT to CF was monitored over 500 hours (Fig. S3, ESI†). Slight band recovery (increment) at 333 nm, and re-splitting of the 299 nm band to 286 nm and 302 nm were observed which could be ascribed to the very slow return to the initial CF. CHR-Hexyl exhibited much slower thermal back transition in toluene compared to that in a mixture of hexane and dichloromethane which was observed from absorption spectra and the chiral HPLC chromatogram (Fig. S2, ESI†). The difference in lifetime of TC and TT depending on the solvent nature has been discussed in a previous report.18
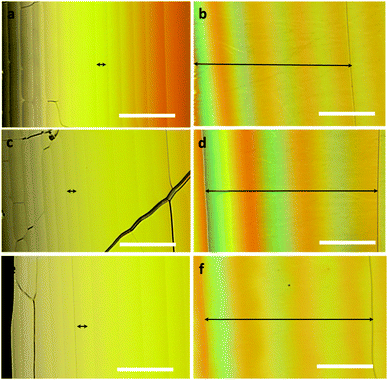
Based on the chiroptical properties, we examined the chirality transfer characteristics of CHR-Hexyl in a nematic liquid crystal medium at room temperature. The chiral nematic liquid crystal composite was prepared by dissolving one of the enantiomers of CHR-HexylM or P and the host liquid crystal such as 4-phenyl-4′-cyanobiphenyl (5CB), JC-1014XX, and E-7. The homogeneous CLC mixture was injected into a wedge cell by capillary force at room temperature, and clear Cano's lines20 were observed under a polarizing optical microscope, which represents the formation of cholesteric orientation of the molecules (Fig. 3 and Fig. S4, ESI†).
Wedge cells filled with the chiral LC mixture containing M showed a color shift towards the thinner region of the wedge upon turning the analyzer (located on the opposite side of the light source with respect to the cell) in a clockwise direction which indicates that the helix is left-handed or bears a negative (−) helical twist. On the other hand, wedge cells with P showed right-handed or (+) twist sense which is opposite to that of M.
The ability of a chiral molecule to induce a helical structure in CLC can be quantified as the helical twisting power (HTP, β) and expressed by the equation β = (PC)−1, where C is the concentration of the chiral dopant, and P is the pitch that corresponds to the distance over which the director of molecules undergoes one full turn.2,3 The HTP and the resultant helical pitch highly depend on the conformational change of the chiral dopant.3,5 The helical pitch length was determined by Cano's wedge method (see the ESI†),20 and its photoinduced variations were evaluated upon UV (365 nm) illumination to the wedge cell which resulted in surprisingly huge widening of the gap between Cano lines for all CLCs as shown in Fig. 3. It took over 1 minute to initiate the lengthening of the distance between Cano's lines by UV light irradiation. This delayed photoresponse would be attributed to the transition between CF and thermodynamically less stable TC-rich states. Based on the obtained helical pitch lengths of CLCs containing CHR-Hexyl (M) and NLC hosts such as JC-1014XX, E7 and 5CB (Fig. 3), each HTP value before (initial) and after UV light irradiation (PSS365nm), and the switching ratio (|Δβ|/βini) were calculated and are described in Table 1.
| NLC | HTP (β), μm−1 | ||
|---|---|---|---|
| Initial | PSS365nm | |Δβ|/βinib (%) | |
| a Determined using Cano's wedge method and the change in HTP under each photoirradiation condition. b Percentage change in HTP observed between the initial state and PSS365nm [(βfin − βini)/|βini|]. | |||
| JC-1014XX | −28.5 | −2.0 | 93.0 |
| E7 | −26.4 | −1.8 | 93.2 |
| 5CB | −20.0 | −1.5 | 92.5 |
To our surprise, a HTP switching ratio of over 90% was attainable, which has never been reported for helicene-like photochromic chiral dopants, and this value was even larger than those of p-type chiral dopants such as azobenzenes and diarylethenes.3,12,21,22 It might originate from the photoisomerization of CHR-Hexyl from CF to TTvia the TC state which leads to a big change in the molecular geometry going from a [6]-helicene to a [5]-helicene like molecule with only four aromatic rings along with the presence of a bulky substituent (dienic unit) at the 1-position. The possibility of racemization induced reduction of HTP could be ruled out as we confirmed negligibly small racemized traces of M and P forms from chiral HPLC after UV light irradiation followed by thermal relaxation (Fig. S2, ESI†). Thus, the extensively perturbed and elongated helical conformation of the TT form results in the reduced chirality transfer to the host medium in accordance with a huge reduction of HTP. The subsequent visible light (510 nm) irradiation at relatively low intensity for 4 hours induced almost no shortening of the pitch length (Fig. S4c, ESI†), while the high-intensity white light irradiation for 1 h resulted in a pitch shortening (HTP increment) ratio of 20% to the initial state (Fig. S5a, ESI†). This result is in agreement with the very low quantum yield (around 0.01) of the photochemical process from TT to CF found in solution. This process should be even slower in a more viscous phase. Pitch length recovery (shortening) of 16% was exhibited by the thermal back reaction of the dopant in 48 hours at room temperature under dark room conditions (Fig. S4d, ESI†) and continuous recovery of the helical pitch length was confirmed for over 6 days (Fig. S5b, ESI†). Unprecedented monotonic HTP increase through helicene ring closure is attributed to the thermodynamically more stable TT form than TC, as revealed from thermal back CD spectra of CHR-Hexyl (Fig. S3, ESI†) and previously reported photophysical study on naphthopyran derivatives.18
Dynamically self-organizable CLCs can be utilized to produce mechanical work from an object using light energy. Several demonstrations have been made based on the collective action of molecular motors in a CLC that can be translated into macroscopic rotational motion of microscale objects.9,12,22–25 It was revealed that the intrinsic degree of rotation of micro-objects is determined by the switching ratio of HTP between before and after light irradiation (|Δβ|βini).25 A maximum photoinduced switching ratio of HTP of 50%,12,22 which requires high dopant concentration to achieve a larger rotation angle, can possibly induce changes in the physical properties of CLCs such as phase separation, viscosity and clearing point. Based on the large HTP switching ratio of over 90% of CHR-Hexyl by UV light irradiation, we attempted to examine the light-induced rotational motion of micro-objects on the surface of its cholesteric mixture.
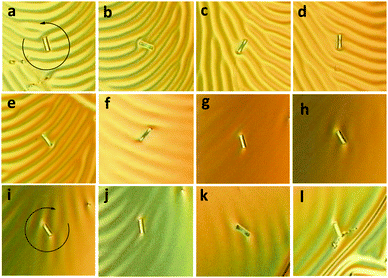
The doped liquid crystalline mixture with CHR-Hexyl (M) enantiomer was drop-cast onto a glass substrate coated with a unidirectionally rubbed polyimide alignment film,12,22 and was then observed under an optical microscope equipped with a polarizer at room temperature. Clear fingerprint textures were observed, which is a direct consequence of the cholesteric geometry, by aligning the axis of the cholesteric helix parallel to the substrate at the interface between the liquid crystal and air. The line width from a polygonal fingerprint texture corresponds to half of the cholesteric pitch (P) length.22 After verifying the formation of polygonal textures, we freckled glass rods (average length: 25 μm; average diameter: 5 μm) onto the film surface and then irradiated the films with light of 365 nm. Fingerprint textures were dynamically reorganized and relaxed, and finally almost disappeared by attaining the TT-rich state of the chiral dopants accompanying the anti-clockwise rotation of the glass rods as shown in Fig. 4 from (a) to (h).
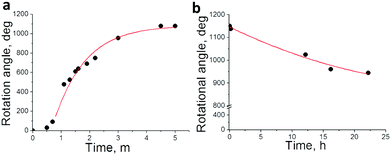
Fig. 5a depicts the rotational angle change depending on the UV irradiation time. Rotation became fast when it passed the threshold at around 40 s, presumably exceeding the boundary irradiation dose of transition from CF–TC to the TT form. It decreased as irradiation continued and eventually stopped due to attainment of the photostationary state of the dopant. Almost 3 full cycles (1150°) were achieved with irradiation for 5 minutes. As a subtle angular change was observed afterwards, the UV light source was removed and the thermal back reaction-induced mechanical motion was monitored at room temperature. Dissipated fingerprint texture gradually reoccurred accompanying the clock-wise rotational motion of glass rods (Fig. 4 from (i) to (l)). The clock-wise rotation angle was 206° in 22 hours, which corresponds to 18% rotation recovery (Fig. 5b). It is highly consistent with the following result: a helical pitch recovery ratio of 16% in 24 hours obtained from the shift of Cano lines (Fig. S4 and S5b, ESI†). This is attributed to the CHR-Hexyl transition from the TT to the CF state which induces the recovery of the helicity of the naphthopyran unit and consequently the HTP increase. Although the maximum rotation angle reached 3.5 cycles (1260°, Fig. S6, ESI†) with longer irradiation (17.5 minutes), neither recovery of fingerprint textures nor reversal rotation was observed from the TT-rich state when left at room temperature. Chiral LC films containing the other enantiomer (P) showed reverse directional rotation compared to that of M as it exhibits an opposite change in HTP.
Rotational angles of microsized objects on the surface of CLCs have been reported with sterically overcrowded alkene motors (900° at 1 wt%),23 central chiral azobenzene (1200° at 6 wt%)24 and planar chiral azobenzene (360° at 0.35 wt%)12 dopants. It is noteworthy that the largest photoinduced rotational angle was achieved with CHR-Hexyl per unit concentration, which is advantageous to provide an exceptionally efficient molecular machinery system. It is ascribed to the transition of the rigid helical geometry (CF) to loosened and elongated helicity (TT) which dramatically reduces chirality transfer of CHR-Hexyl resulting in a large HTP switching ratio of over 90%. Moreover, the high thermal stability of the TT form after UV irradiation resulted in very slow backward rotation, which has never been demonstrated so far, and possibly led to the much precise control including the bistable memory of rotation angles.
In conclusion, we demonstrated photoresponsive CLCs comprising a photoisomerizable naphthopyran dopant for the first time exhibiting both dynamic helical reorganization performance and unique high thermal stability by CF–TT isomerization. It is highly expected that the novel system can be exploited to provide bistable phototunability of mechanical work.
This work was supported by the Toyo Gosei Memorial Foundation and JSPS KAKENHI Grant Number JP16K17886.
Notes and references
- B. L. Feringa and W. R. Browne, Molecular Switches, Wiley-VCH, Germany, 2011 Search PubMed.
- H. K. Bisoyi and Q. Li, Angew. Chem., Int. Ed., 2016, 55, 2994 CrossRef CAS PubMed.
- R. Eelkama and B. L. Feringa, Org. Biomol. Chem., 2006, 4, 3729 Search PubMed.
- S. Chandrasekhar, Liquid Crystals, Cambridge University Press, Cambridge, UK, 1992 Search PubMed.
- S. Pieraccini, S. Masiero, A. Ferrarini and G. P. Spada, Chem. Soc. Rev., 2011, 40, 258 RSC.
- Q. Li, Photoresponsive Cholesteric Liquid Crystals, Intelligent Stimuli-Responsive Materials: From Well-Defined Nanostructures to Applications, John Wiley & Sons, 2013 Search PubMed.
- Z. Zheng, Y. Li, H. K. Bisoyi, L. Wang, T. J. Bunning and Q. Li, Nature, 2016, 531, 352 CrossRef CAS PubMed.
- S. Abraham, V. A. Mallia, K. V. Ratheesh, N. Tamaoki and S. Das, J. Am. Chem. Soc., 2006, 128, 7692 CrossRef CAS PubMed.
- R. Eelkema, M. M. Pollard, J. Vicario, N. Katsonis, B. S. Ramon, C. W. M. Bastiaansen, D. J. Broer and B. L. Feringa, Nature, 2006, 440, 123 CrossRef PubMed.
- N. Tamaoki, Adv. Mater., 2001, 13, 1135 CrossRef CAS.
- S. Furumi and N. Tamaoki, Adv. Mater., 2010, 22, 886 CrossRef CAS PubMed.
- Y. Kim and N. Tamaoki, J. Mater. Chem. C, 2014, 2, 9258 RSC.
- Y. Kim, M. Wada and N. Tamaoki, J. Mater. Chem. C, 2014, 2, 1921 RSC.
- S. Tokunaga, Y. Itoh, Y. Yaguchi, H. Tanaka, F. Araoka, H. Takezoe and T. Aida, Adv. Mater., 2016, 28, 4077 CrossRef CAS PubMed.
- A. Ferrarini, S. Pieraccini, S. Masiero and G. P. Spada, Beilstein J. Org. Chem., 2009, 5, 1 Search PubMed.
- G. Gottarelli, G. Proni, G. P. Spada, D. Fabbri, S. Gladiali and C. Rosini, J. Org. Chem., 1996, 61, 2013 CrossRef CAS.
- M. Frigoli, F. Maurel, J. Berthet, S. Delbaere, J. Marrot and M. M. Oliveira, Org. Lett., 2012, 14, 4150 CrossRef CAS PubMed.
- M. Frigoli, J. Marrot, P. L. Gentili, D. Jacquemin, M. Vagnini, D. Pannacci and F. Ortica, ChemPhysChem, 2015, 16, 2447 CrossRef CAS PubMed.
- T. Kosa, L. Sukhomlinova, L. Su, B. Taheri, T. J. White and T. J. Bunning, Nature, 2012, 485, 347 CrossRef CAS PubMed.
- R. Cano, Bull. Soc. Fr. Mineral., 1968, 91, 20 CAS.
- Y. Li, M. Wang, H. Wang, A. Urbas and Q. Li, Chem. – Eur. J., 2014, 20, 16286 CrossRef CAS PubMed.
- Y. Kim and N. Tamaoki, ACS Appl. Mater. Interfaces, 2016, 8, 4918 CAS.
- R. Eelkema, M. M. Pollard, N. Katsonis, J. Vicario, D. J. Broer and B. L. Feringa, J. Am. Chem. Soc., 2006, 128, 14397 CrossRef CAS PubMed.
- A. Kausar, H. Nagano, Y. Kuwahara, T. Ogata and S. Kurihara, Chem. – Eur. J., 2011, 17, 508 CrossRef CAS PubMed.
- R. Thomas, Y. Yoshida, T. Akasaka and N. Tamaoki, Chem. – Eur. J., 2012, 18, 12337 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Chiral HPLC chromatograms; thermal back CD spectra of CHR-Hexyl solution; cholesteric pitch relaxation; and images of the rotational motion of a micro glass rod on a CLC reaching PSS365nm. See DOI: 10.1039/c6cc08667e |
| This journal is © The Royal Society of Chemistry 2017 |