Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Biofunctional metal–phenolic films from dietary flavonoids†
Nadja
Bertleff-Zieschang
,
Md. Arifur
Rahim
 ,
Yi
Ju
,
Yi
Ju
 ,
Julia A.
Braunger
,
Tomoya
Suma
,
Yunlu
Dai
,
Shuaijun
Pan
,
Francesca
Cavalieri
and
Frank
Caruso
,
Julia A.
Braunger
,
Tomoya
Suma
,
Yunlu
Dai
,
Shuaijun
Pan
,
Francesca
Cavalieri
and
Frank
Caruso
 *
*
Australian Research Council (ARC) Centre of Excellence in Convergent Bio-Nano Science and Technology, and the Department of Chemical and Biomolecular Engineering, The University of Melbourne, Parkville, Victoria 3010, Australia. E-mail: fcaruso@unimelb.edu.au
First published on 3rd January 2017
Abstract
We assembled dietary, bioactive flavonoids into a metal coordinated network to form thin, surface-bound films and hollow capsules, overcoming the poor water solubility of free flavonoids. Films formed from quercetin, myricetin, luteolin and fisetin show radical scavenging activity, a renowned feature of their parent flavonoids, and can be reused over multiple cycles. These films are expected to have potential applications in the pharmaceutical and food industries.
Dietary flavonoids constitute an important class of plant polyphenols and are present in abundance in fruits, vegetables and leaves, where they fulfill diverse biological functions from pigmentation to defense against chemical and radiation damage.1 Due to their human health benefits flavonoids have gained increasing attention for applications in anticancer treatment, protection against cardiovascular disease, and mitigation of inflammation.2,3 A drawback of most flavonoids is their poor water solubility. Strategies to address this issue range from encapsulation of flavonoids into liposome or polymeric carriers4 to synthetic modifications.5,6 However, the preparation of thin surface-bound or free-standing films from dietary flavonoids has largely remained unexplored.
Here, we report the one-step assembly of four different flavonoids upon coordination with FeIII ions into thin films on solid surfaces. Considerable effort has been given to the fabrication of bioinspired functional coatings for application in medicine, optics and catalysis.7,8 One prominent example is polyphenolic films obtained via oxidative polymerization on templating surfaces.9,10 We recently reported a coordination-driven approach to deposit thin amorphous films from a variety of phenolic ligands and metal ions.11–13 Among the different ligands pyrocatechol (PC, ortho-dihydroxybenzene) was found to be the simplest phenolic ligand that assembles with FeIII into a metal phenolic network (MPN).13 Given that the ortho-dihydroxyphenyl group is a common structural feature of many bioactive flavonoids, we investigated whether flavonoids could be assembled into nanostructured coatings and free-standing films, as such materials could be of potential interest in the pharmaceutical and food industries.
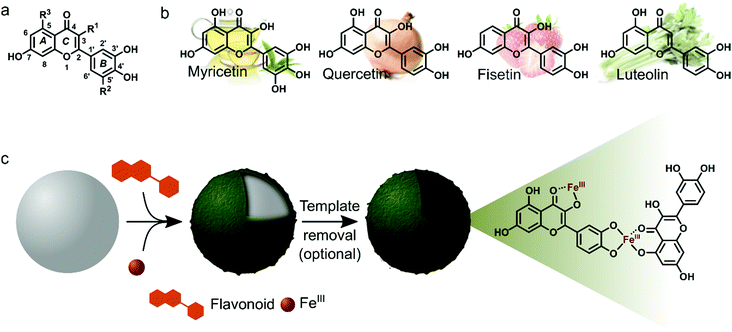
Myricetin (Myr), quercetin (Que), fisetin (Fis) and luteolin (Lut) are well known for their antioxidant, anti-inflammatory and antibacterial properties. Due to these properties we were interested in studying the MPN formation of flavonoid/FeIII films and to analyze the resulting films regarding their biofunctionality. The flavonoids in this study share a common structural feature, the ortho-dihydroxyphenyl group, but the overall hydroxylation pattern varies from Myr to Lut. Scheme 1 depicts the molecular structures of the flavonoids and illustrates the preparation of surface-bound films and hollow capsules that can be obtained after template removal.
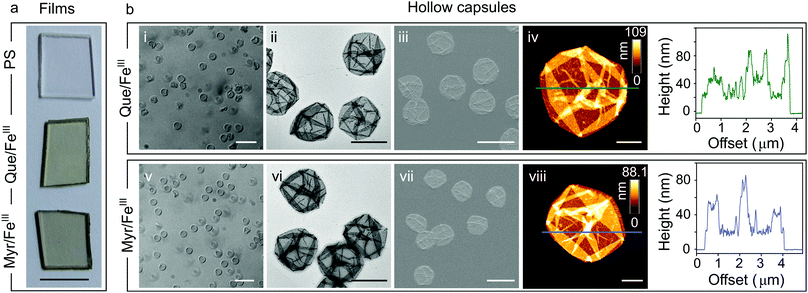
For characterization of flavonoid MPN films we focused on the systems Que/FeIII and Myr/FeIII. Simple mixing of the flavonoids with FeIII in the presence of solid substrates instantaneously produces colored films (Fig. 1a and Fig. S1, ESI†). Well-dispersed hollow capsules were obtained from particulate substrates after template removal (e.g. PS microparticles, Fig. 1bi and v). The zeta (ζ)-potentials of Que/FeIII and Myr/FeIII capsules were found to be −26 ± 3 mV and −34 ± 3 mV, respectively, most likely because of the acidic hydroxyl groups in both flavonoids (pKa1 = 7.2 (Que),14 pKa1 = 6.6 (Myr)15). ζ-Potentials are in the range for colloidally stable particles.16 Que/FeIII and Myr/FeIII capsules did not agglomerate in aqueous solution and intact capsules were observed for both systems over 10 days (Fig. S2, ESI†). Considering the molecular structure of Que and Myr, network formation can be assumed to occur via several coordination sites. Que and Myr are flavonol-type flavonoids consisting of a 3-ring system with two aromatic rings (A and B) that are linked via a heterocyclic pyrane ring C (Scheme 1a).17 Among the different aromatic hydroxyl groups, the 3′,4′-dihydroxyphenyl group has been reported to show the highest affinity for FeIII.18,19 Further coordination sites within the flavonols are presented by the carbonyl oxygen with either the 3-hydroxyl or 5-hydroxyl group.18,19 The different reported coordination modes of Que to FeIII are illustrated in Scheme 1c.
To further investigate metal coordination in the networks, UV/Vis absorption spectroscopy was performed on the Que/FeIII and Myr/FeIII capsules. UV/Vis absorption spectra of the Que/FeIII and Myr/FeIII systems showed two major absorption bands, each at 271 and 436 nm, and 265 and 437 nm, respectively (Fig. S3, ESI†). In contrast, free flavonoids showed absorption bands at shorter wavelengths (256 and 374 nm, and 254 and 378 nm for Que and Myr, respectively, Fig. S3, ESI†), which can be assigned to the π–π* transition of the A ring (benzoyl system, band II) and the B ring (cinnamoyl system, band I), respectively (Scheme 1a).17 A bathochromic shift upon metal coordination has been observed for the Que absorption band I previously17,20 and has been ascribed to the extension of the conjugated π-system rather than to a ligand to metal charge transfer (LMCT)17 characteristic of catechol–FeIII complexes.21 This indicates that metal coordination at the B ring is the major driving force for film formation. Additionally, fluorescence spectroscopy of the Que/FeIII system revealed that the emission of Que at 517 nm (λexc = 380 nm) is efficiently quenched by FeIII coordination (Fig. S4, ESI†), which is consistent with previous reports on Que complexes with CuII, FeII and FeIII.22,23
Next, we analyzed the morphology and composition of air-dried capsules by transmission electron microscopy (TEM, Fig. 1bii and vi), scanning electron microscopy (SEM, Fig. 1biii and vii), and atomic force microscopy (AFM, Fig. 1biv and viii). AFM height analysis yielded single wall thicknesses of 12.0 ± 0.6 nm and 13.2 ± 1.1 nm for Que/FeIII and Myr/FeIII capsules, respectively, which are similar to values obtained for tannic acid(TA)/FeIII (10.4 ± 0.6 nm)11 and PC/FeIII (11.4 ± 0.4 nm)13 capsules in previous work. Surface roughness analyses resulted in values of 3.6 ± 0.4 nm and 2.3 ± 0.6 nm for Que/FeIII and Myr/FeIII capsules, respectively, which are higher than found for TA/FeIII (1.6 ± 0.1 nm)11 and PC/FeIII (ca. 0.4 nm).13 X-ray photoelectron spectroscopy (XPS) on Que/FeIII and Myr/FeIII capsules indicated that FeIII is the predominant species in the films with Fe 2p3/2 and Fe 2p1/2 signals at ∼712 eV and ∼725 eV (Fig. S5, ESI†).24 Energy-dispersive X-ray (EDX) spectroscopy and elemental mapping further showed a uniform distribution of Fe, C and O within the capsules (Fig. S6 and S7, ESI†) of Que/FeIII and Myr/FeIII.
The underlying mechanism of the network formation is a pH-dependent process based on coordination chemistry between a flavonoid ligand and FeIII. At acidic pH, Que/FeIII and Myr/FeIII capsules readily disassembled, as monitored by optical microscopy. UV/Vis spectroscopy of the disassembled Que/FeIII system further revealed the disappearance of absorption bands assigned to the complex and the reappearance of bands at 256 and 374 nm found in free Que (Fig. S3a, ESI†). Interestingly, the absorption bands in the UV/Vis spectrum of acid-treated Myr/FeIII capsules did not match with those of free Myr, suggesting that Myr at least partly coordinates to FeIII under these conditions (Fig. S3b, ESI†). Similar to the UV/Vis spectroscopy findings, the fluorescence emission observed for free Que was restored when disassembling Que/FeIII capsules (Fig. S4, ESI†). The on/off fluorescence between capsules and free Que is potentially promising for application in sensing and monitoring of Que release in a physiological context. In addition, pH-induced disassembly of Que/FeIII capsules served to quantitatively determine the amount of flavonoid and metal in a single capsule. The capsule concentration was first determined by flow cytometry and the amount of Que was subsequently estimated to be 0.67 fmol per capsule from UV/Vis spectroscopy of the acid-treated sample. Inductively coupled plasma optical emission spectroscopy (ICP-OES) experiments yielded a Fe concentration of ca. 0.23 fmol per capsule, leading to a ligand/metal ratio of ∼3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
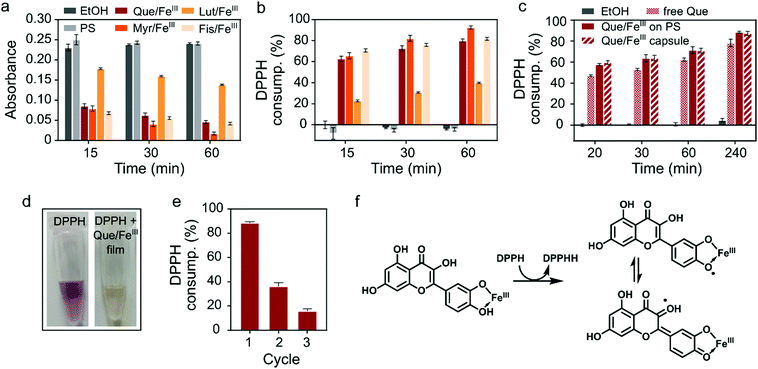
Finally, we investigated the biological activity of flavonoid/FeIII films. Among the plethora of functions attributed to dietary flavonoids the most renowned are their antioxidant properties and their ability to scavenge free radicals.25 Radical oxygen species (ROS) play critical roles in inflammation processes. Therefore, films with radical scavenging properties have potential as cytoprotective and anti-inflammatory coatings of, for example, medical implants.9,26,27 We probed the radical scavenging activity of the flavonoid/FeIII coatings with the well-established method based on the stable 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical, which shows a characteristic absorption at 517 nm in ethanol.9,28 Here, radical scavenging activity can be visually followed by discoloration of the purple ethanolic solution of DPPH (Fig. 2d) and quantified by UV/Vis spectroscopy. The decrease in absorption at 517 nm (Fig. 2a) can be correlated to the consumption of the DPPH radical (molecular structures of the DPPH radical and the reduced DPPHH are shown in Fig. S8, ESI†). Films from Que, Myr and Fis showed strong scavenging activity with 80% or more DPPH consumption after 60 min (Fig. 2b), while the Lut/FeIII network consumed less than 40% (Fig. 2b), presumably due to the absence of the 3-hydroxyl group in the C ring.29,30 Based on this finding, we compared the scavenging activity of free Que, Que/FeIII coatings and Que/FeIII capsules of the same Que concentration (Fig. 2c). Both Que/FeIII films on PS particles and as capsules consumed the same amount of DPPH, as monitored over 4 h, which indicates that PS only acts as a template and does not interfere with the scavenging activity of the MPN layer. Further, Que/FeIII films showed ca. 10% higher activity than free Que. The higher scavenging activity of complexed flavonoids can be explained by the stabilization of the intermediate semiquinone radical by the iron center and the conjugation with the 3-OH group (Fig. 2f).20,30 In addition to the higher scavenging activity of our Que/FeIII films, we found that the films can be reused and that the scavenging activity was lowered but preserved over at least three cycles (Fig. 2e). Que collected from the films that were exposed to DPPH for 30 min showed a decrease in the absorption band of the B ring at 374 nm while a new band appeared at 292 nm (Fig. S9, ESI†). We assume that oxidation of the films by DPPH decreases the amount of available antioxidant species and reduces the scavenging activity in the subsequent cycles. Taken together, the higher scavenging activity of the flavonoid/FeIII films, and the easy removal and reusability of coated particles provides advantages over free flavonoids.
In conclusion, we developed biofunctional films and capsules from dietary flavonoids (Que, Myr, Lut, and Fis), exploiting the rapid coordination-driven assembly with FeIII. Flavonoids have many attractive properties in a biomedical context, including antioxidant, anti-inflammatory, anticancer and antimicrobial activity. However, most flavonoids suffer from poor water solubility, limiting their therapeutic usefulness. We overcame this issue by making flavonoids available in thin metal-coordinated films with radical scavenging activity. We demonstrated that the antioxidant activity of the complexed Que is higher than that of the free molecule and is preserved over multiple scavenging cycles. The versatility and simplicity of this approach makes flavonoid MPN films and capsules attractive candidates for potential applications in biomedicine and the food industry. Part of our future studies is to investigate other metal ions to form flavonoid MPNs to combine the properties of flavonoids with those of different metal ions. For example, we have demonstrated previously that MPNs from tannic acid and biomedically relevant metals, such as GdIII, 64CuII and EuIII, show potential for diagnostics and bio-imaging.12
This research was conducted and funded by the Australian Research Council Centre of Excellence in Convergent Bio-Nano Science and Technology (project number CE140100036). This work was also supported by the Australian Research Council (ARC) under the Australian Laureate Fellowship (F. Caruso, FL120100030), Super Science Fellowship (F. Caruso, FS110200025), and Future Fellowship (F. Cavalieri, FT140100873) schemes. This work was performed in part at the Materials Characterisation and Fabrication Platform (MCFP) at the University of Melbourne and the Victorian Node of the Australian National Fabrication Facility (ANFF).
Notes and references
- F. Ververidis, E. Trantas, C. Douglas, G. Vollmer, G. Kretzschmar and N. Panopoulos, Biotechnol. J., 2007, 2, 1214–1234 CrossRef CAS PubMed.
- A. García-Lafuente, E. Guillamón, A. Villares, M. A. Rostagno and J. A. Martínez, Inflammation Res., 2009, 58, 537–552 CrossRef PubMed.
- M.-H. Pan, C.-S. Lai and C.-T. Ho, Food Funct., 2010, 1, 15–31 CAS.
- F. Aqil, R. Munagala, J. Jeyabalan and M. V. Vadhanam, Cancer Lett., 2013, 334, 133–141 CrossRef CAS PubMed.
- M. K. Kim, H. Choo and Y. Chong, J. Med. Chem., 2014, 57, 7216–7233 CrossRef CAS PubMed.
- H. Zhang, M. Zhang, L. Yu, Y. Zhao, N. He and X. Yang, Food Chem. Toxicol., 2012, 50, 1589–1599 CrossRef CAS PubMed.
- Y. L. Liu, K. L. Ai and L. H. Lu, Chem. Rev., 2014, 114, 5057–5115 CrossRef CAS PubMed.
- M. Krogsgaard, V. Nue and H. Birkedal, Chem. – Eur. J., 2015, 22, 844–857 CrossRef PubMed.
- T. S. Sileika, D. G. Barrett, R. Zhang, K. H. A. Lau and P. B. Messersmith, Angew. Chem., Int. Ed., 2013, 52, 10766–10770 CrossRef CAS PubMed.
- T. S. Sileika, H.-D. Kim, P. Maniak and P. B. Messersmith, ACS Appl. Mater. Interfaces, 2011, 3, 4602–4610 CAS.
- H. Ejima, J. J. Richardson, K. Liang, J. P. Best, M. P. van Koeverden, G. K. Such, J. Cui and F. Caruso, Science, 2013, 341, 154–157 CrossRef CAS PubMed.
- J. Guo, Y. Ping, H. Ejima, K. Alt, M. Meissner, J. J. Richardson, Y. Yan, K. Peter, D. von Elverfeldt, C. E. Hagemeyer and F. Caruso, Angew. Chem., Int. Ed., 2014, 53, 5546–5551 CrossRef CAS PubMed.
- M. A. Rahim, K. Kempe, M. Müllner, H. Ejima, Y. Ju, M. P. van Koeverden, T. Suma, J. A. Braunger, M. G. Leeming, B. F. Abrahams and F. Caruso, Chem. Mater., 2015, 27, 5825–5832 CrossRef CAS.
- J. M. Herrero-Martínez, M. Sanmartin, M. Rosés, E. Bosch and C. Ràfols, Electrophoresis, 2005, 26, 1886–1895 CrossRef PubMed.
- Y. S. Yao, G. B. Lin, Y. Xie, P. Ma, G. W. Li, Q. C. Meng and T. Wu, Pharmazie, 2014, 69, 19–26 CAS.
- Zetasizer Nano Series User Manual, Feb. 2004, MAN0317.
- M. Guo, C. Perez, Y. Wei, E. Rapoza, G. Su, F. Bou-Abdallah and N. D. Chasteen, Dalton Trans., 2007, 4951–4961 RSC.
- G. M. Escandar and L. F. Sala, Can. J. Chem., 1991, 69, 1994–2001 CrossRef CAS.
- M. D. Engelmann, R. Hutcheson and I. F. Cheng, J. Agric. Food Chem., 2005, 53, 2953–2960 CrossRef CAS PubMed.
- R. F. V. de Souza and W. F. De Giovani, Redox Rep., 2004, 9, 97–104 CrossRef CAS PubMed.
- N. R. Perron and J. L. Brumaghim, Cell Biochem. Biophys., 2009, 53, 75–100 CrossRef CAS PubMed.
- S. Yang, B. Yin, L. Xu, B. Gao, H. Sun, L. Du, Y. Tang, W. Jiang and F. Cao, Anal. Methods, 2015, 7, 4546–4551 RSC.
- C. C. Yu and Z. H. Cheng, Anal. Lett., 2003, 36, 767–780 CrossRef.
- T. Yamashita and P. Hayes, Appl. Surf. Sci., 2008, 254, 2441–2449 CrossRef CAS.
- G. B. Bubols, D. D. Vianna, A. Medina-Remon, G. von Poser, R. M. Lamuela-Raventos, V. L. Eifler-Lima and S. C. Garcia, Mini-Rev. Med. Chem., 2013, 13, 318–334 CAS.
- M. M. Kasprzak, A. Erxleben and J. Ochocki, RSC Adv., 2015, 5, 45853–45877 RSC.
- I. B. Afanas'ev, E. A. Ostrakhovitch, E. V. Mikhal'chik, G. A. Ibragimova and L. G. Korkina, Biochem. Pharmacol., 2001, 61, 677–684 CrossRef.
- Y. Deligiannakis, G. A. Sotiriou and S. E. Pratsinis, ACS Appl. Mater. Interfaces, 2012, 4, 6609–6617 CAS.
- Y. Kwon, H. Kim, S. Park and S. Jung, Bull. Korean Chem. Soc., 2010, 31, 3035–3037 CrossRef CAS.
- M. Y. Moridani, J. Pourahmad, H. Bui, A. Siraki and P. J. O'Brien, Free Radical Biol. Med., 2003, 34, 243–253 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cc08607a |
| This journal is © The Royal Society of Chemistry 2017 |