Hue-based quantification of mechanochromism towards a cost-effective detection of mechanical strain in polymer systems†
A.
Battisti
 ab,
P.
Minei
c,
A.
Pucci
ab,
P.
Minei
c,
A.
Pucci
 *c and
R.
Bizzarri
*ab
*c and
R.
Bizzarri
*ab
aNEST – Scuola Normale Superiore, Istituto Nanoscienze – CNR (CNR-NANO), p.zza San Silvestro 12, 56127 Pisa, Italy. E-mail: ranieri.bizzarri@nano.cnr.it
bM3-Village, Consorzio Interuniversitario Nazionale per la Scienza e Tecnologia dei Materiali – INSTM, p.zza San Silvestro 12, 56127 Pisa, Italy
cDipartimento di Chimica e Chimica Industriale, Università di Pisa, via Moruzzi 13, 56124 Pisa, Italy. E-mail: andrea.pucci@unipi.it
First published on 25th November 2016
Abstract
HSV is a digital colour space easily accessible by the transformation of RGB images. In this work, the hue parameter H was used to assess mechanically induced colour changes of the aggregation-sensitive fluorescent dye 4,4′-bis-(2-benzoxazolyl)stilbene (BBS), thus implementing a cheap and reliable method for the detection of mechanical deformation in a polymer matrix.
Colorimetric sensors are widely employed not only in the detection and quantification of analytes such as gases,1,2 anions,3,4 cations,5,6 protons,7 biomolecules,8,9 but also in the detection of other stimuli such as deformation,10 temperature alterations,11,12 light exposure,13–15etc. The basic principle underlying the use of such sensors is the predictable relationship between the presence of the relevant analyte or stimulus and changes in the wavelength of light absorbed and/or emitted by the sensing molecule.
Among colorimetric fluorescent sensors, it is worth citing the fluorescent dye 4,4′-bis-(2-benzoxazolyl)stilbene (BBS, Scheme 1). BBS is a stilbene derivative typically employed as an optical brightener in several polymers, thanks to its high compatibility with the continuous matrices.16 Moreover, the high resistance of this fluorophore to solvent extraction and its very high melting point (360 °C) and degradation temperature (380 °C) comply with the US Food and Drug Administration (FDA) regulations, making it ideal as an additive for food packaging materials.16 Its fluorescence response depends on the presence of isolated chromophores, which emit in the blue region, or aggregates accounting for the formation of an excimer-type emission band centred in the green region;17,18 the deformation of BBS-loaded polymeric films (which induces disassembly of BBS aggregates) can then be followed by monitoring the fluorescence colour change, which makes BBS an excellent sensor for smart-material applications. The first example of aggregachromic dyes dispersed in a polymer matrix was reported by Weder et al., who paved the way for the realization of mechanochromic films based on melt-processed polymer mixtures.19 The dispersion of a dye into a polymer is commonly employed in commodity plastics and the procedure has been used for pigmented materials since the early days of the plastic era.20 The chances to exploit the features of mechanochromism are based on the availability of fast, sensitive and cost-effective detecting techniques. In this context, we reasoned that the current widespread use of digital devices equipped with RGB cameras might provide a low-cost alternative to conventional wavelength-discriminating instruments relying on the sequential use of optical discriminators such as filters and monochromators. Accordingly, we disclose a simple and inexpensive method for the detection of colour changes in the fluorescence of BBS-loaded polymers upon deformation. Remarkably, this approach is not limited to BBS, but it could be applied to a wide range of colorimetric systems, spanning from materials science to industrial quality control.
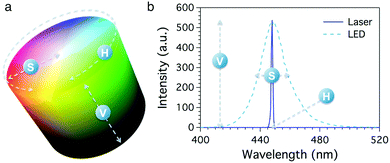
Recently, Hakonen et al. demonstrated the possible use of HSV (Hue, Saturation and Value) colour space to monitor colour changes in a pH-sensitive sensor.21 Indeed, digital images can be converted to several formats depending on the kind of information that has to be collected. HSV (also known as HSB, Hue, Saturation and Brightness) is a digital colour format where the colour of each pixel is identified by the coordinates of its position in a cylindrical space (Fig. 1a). The three different coordinates are hue (H), which represents the colour tone expressed as an angular value ranging from 0° to 360° around the cylinder circumference, saturation (S), which ranges from 100% (pure colour) to 0% (grey) and locates the point along the cylinder radius, and value (V), which consists in the maximum intensity of the signal given by the pixel, locating the pixel along the cylinder axis. The mathematical piecewise equations used to obtain the HSV values from an RGB image are reported in the ESI.† It is worth noting that neutral colours (greys) are undefined in this space; actually, the grey scale is given by any colour showing a 0% value for S and a V value ranging from 0% (black) to 100% (white).22
Remarkably, the HSV coordinates are intimately connected with the physical properties of collected light. Considering the light spectrum, H reflects the emission wavelength, S the bandwidth of the peak and V its height. Fig. 1b shows the emission spectra of two different blue light sources, i.e. a laser source and a LED lamp. In this example, the two sources show same emission intensity (V) and wavelength (H) but different peak width (S); the laser light emission profile (solid line) shows a very narrow band, corresponding to a nearly pure colour, while the LED lamp (dashed line) produces a broad band, reflecting a lower saturation value.
Cantrell et al. demonstrated how RGB images collected with any digital device can be easily converted to the relevant HSV stack and compared in terms of colour hue H without artefacts due to different illumination conditions, which would affect only the V and S parameters.23 Accordingly, the emission wavelength of a colorimetric fluorescent sensor is to be precisely identified by a given hue value, and this feature has been exploited in the sensing of pH21 and ions,23,24 and to monitor the degradation of porous silicon photonic crystals.25
Following this approach, BBS was homogeneously dispersed in a linear low-density polyethylene (LLDPE) matrix. LLDPE is a mostly linear polymer that differs from low-density polyethylene (LDPE) in the absence of long side chains. LLDPE shows higher resistance to impact and puncture and it easily extends under stress. It finds broad applications in the production of thin films for stretch cling sheets, plastic bags, silage films, and plastic wraps. LLDPE films containing 0.3 wt% of BBS were prepared by compression moulding of the relevant dye/polymer mixtures, obtained by melt blending the components in a discontinuous Brabender type mixer. After removal from the press, the BBS/LLDPE films were allowed to cool down to room temperature (see the ESI† for further details). The films were then cut in pieces of 1 × 1 cm and stretched according to definite stretching ratios, from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (not stretched) to 6
1 (not stretched) to 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (i.e. six times the original length). Stretching ratios were calculated as the ratio between the final and the original distance separating two ink-marks printed on the films before stretching. The films were optically characterized by fluorescence spectroscopy (ESI†) as reported in Fig. 2a.
1 (i.e. six times the original length). Stretching ratios were calculated as the ratio between the final and the original distance separating two ink-marks printed on the films before stretching. The films were optically characterized by fluorescence spectroscopy (ESI†) as reported in Fig. 2a.
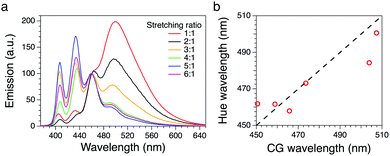 | ||
| Fig. 2 (a) Fluorescence spectra of the BBS/LLDPE films with different stretching ratios (λexc = 360 nm). (b) Wavelength calculated from the hue calibration line vs. CG wavelength. | ||
To evaluate the possibility to follow the stretching process by means of hue variations in the HSV colour space, digital images of the samples were taken under UV irradiation at 366 nm using the on-board camera of a Nokia Lumia 635 mobile phone (see details in the ESI†). Pictures were imported in open access software ImageJ (National Institute of Health, Bethesda, MD, USA; available for download at http://https://imagej.nih.gov/ij/) and converted to HSV colour space by means of the embedded native plug-in, thus obtaining a stack of three layers (H, S and V) for each picture. Average H values were obtained from selected areas of the H layer and rescaled to span the 0–360° range. The S and V layers were used to evaluate the homogeneity of the picture and to select the region of interest (ROI).
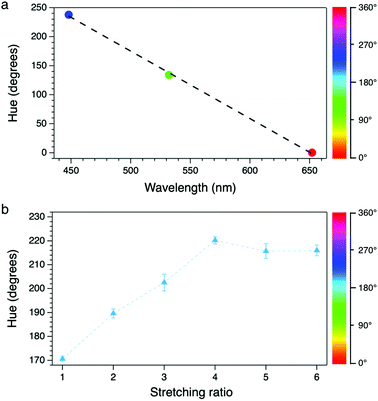
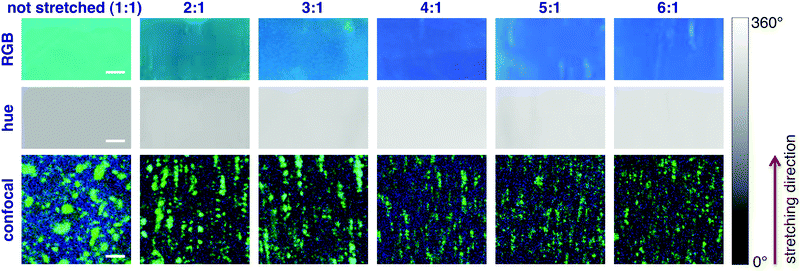
The system was first calibrated by taking pictures of the light cast by three reference laser sources (red, green and blue) emitting at 652, 532 and 448 nm, respectively. The conversion to HSV gave average hue values of 0.24, 134 and 238°; data were fitted obtaining a good linearity between the hue and the wavelength (Fig. 3a). Pictures of the stretched polymeric films were then taken under UV irradiation at 366 nm; confocal fluorescence imaging (ESI†) was performed on the same samples to monitor changes in their microstructure. Fig. 4 shows the comparison between the original RGB images (top row), the hue layer relevant to the same ROI after the conversion to HSV (middle row) and images collected using confocal fluorescence microscopy (bottom row). The first sample (not stretched) shows an average hue value of about 170°; this value linearly increases with stretching, reaching a plateau around 216° after a four-time stretching ratio (Fig. 3b). This behaviour is consistent with the spectroscopic data, since the main peak found for the untreated sample, centred at 500 nm, decreases with stretching, along with the increase of the two peaks in the blue region (Fig. 2a). We note that the emission spectra of the samples are complex, resulting from the overlapping of several peaks; to visualize a correspondence between the hue and the emission spectra, the spectral centre of gravity (CG, for calculation see the ESI†) was plotted against the wavelength corresponding to each hue value according to the calibration line, and a good agreement between the two wavelengths was observed as expected (Fig. 2b).
Notably, colour changes in the samples reflect alterations in the microscopic structure of BBS/LLDPE films due to stretching, as shown by confocal images in the bottom row of Fig. 4: along with the increase in the stretching ratio, the larger BBS clusters are disrupted while smaller elongated aggregates appear, preferentially oriented along the stretching direction. Since this effect is visible only at the microscale, images taken by the camera turn out to be homogeneous, especially when the hue layer is extracted (Fig. 4, middle row); indeed, average light intensity is collected from fairly large areas of the films. The progressive disappearance of large green-emitting aggregates in favour of the blue-emitting monomer accounts for the emission spectrum modification and for the resulting colour change.
It is worth highlighting that, in spite of the complexity of the emission spectra (which span from 400 to 600 nm, i.e. from violet to red), monotonic changes in the position of the centre of gravity can be precisely followed thanks to the hue method. This holds true for those cases where many emission wavelengths are present, provided that the process under investigation causes a monotonic shift in the emission CG.23 Limits arise when multiple variations occurring simultaneously compensate each other to some extent; colour alteration in this case could be undistinguishable or not directly attributable to the monitored process, owing to the unpredictable spectral composition.
In conclusion, the hue parameter allows for the extraction of spectral information from digital colour images without the need for wavelength discriminators. In addition to the possible applications in the readout of bitonal sensors, as long as the variation in the emission spectrum induced by the monitored process is expressible as a continuous function, the hue value can also be used to dig out quantitative information from the qualitative parameter (colour). Indeed, hue evaluation on polymeric films loaded with the mechanochromic dye BBS easily returns the degree of mechanical strain in the polymer. This approach turns out to be valuable as a cost-effective and sensitive tool for several applications such as smart and intelligent packaging, anti-counterfeiting, and quality control systems. We may also envision mobile applications able to calculate in real-time the average hue value of the sample visualized on the device screen by the integrated camera.
Notes and references
- N. A. Rakow and K. S. Suslick, Nature, 2000, 406, 710 CrossRef CAS PubMed.
- P. Minei, M. Koenig, A. Battisti, M. Ahmad, V. Barone, T. Torres, D. M. Guldi, G. Brancato, G. Bottari and A. Pucci, J. Mater. Chem. C, 2014, 2, 9224 RSC.
- H. Maeda and P. Anzenbacher, Supramolecular Chemistry, John Wiley & Sons, Ltd, 2012 Search PubMed.
- R. M. Duke, E. B. Veale, F. M. Pfeffer, P. E. Kruger and T. Gunnlaugsson, Chem. Soc. Rev., 2010, 39, 3936 RSC.
- N. Kaur and S. Kumar, Tetrahedron, 2011, 67, 9233 CrossRef CAS.
- G. Chen, Z. Guo, G. Zeng and L. Tang, Analyst, 2015, 140, 5400 RSC.
- G. Liebsch, I. Klimant, C. Krause and O. S. Wolfbeis, Anal. Chem., 2001, 73, 4354 CrossRef CAS PubMed.
- M. J. Antony and M. Jayakannan, Langmuir, 2011, 27, 6268 CrossRef CAS PubMed.
- Y. Zhou, Z. Xu and J. Yoon, Chem. Soc. Rev., 2011, 40, 2222 RSC.
- F. Ciardelli, G. Ruggeri and A. Pucci, Chem. Soc. Rev., 2013, 42, 857 RSC.
- A. Seeboth, D. Lötzsch, R. Ruhmann and O. Muehling, Chem. Rev., 2014, 114, 3037 CrossRef CAS PubMed.
- C. E. Sing, J. Kunzelman and C. Weder, J. Mater. Chem., 2009, 19, 104 RSC.
- M. Qin, Y. Huang, F. Li and Y. Song, J. Mater. Chem. C, 2015, 3, 9265 RSC.
- P. Minei, A. Battisti, S. Barondi, M. Lessi, F. Bellina, G. Ruggeri and A. Pucci, ACS Macro Lett., 2013, 2, 317 CrossRef CAS.
- F. Ercole, T. P. Davis and R. A. Evans, Polym. Chem., 2010, 1, 37 RSC.
- D. A. Jervis, Plast. Addit. Compd., 2003, 5, 42 CAS.
- M. A. Fourati, W. G. Skene, C. G. Bazuin and R. E. Prud'homme, J. Phys. Chem. A, 2013, 117, 836 CrossRef CAS PubMed.
- A. Pucci, F. Di Cuia, F. Signori and G. Ruggeri, J. Mater. Chem., 2007, 17, 783 RSC.
- B. R. Crenshaw and C. Weder, Chem. Mater., 2003, 15, 4717 CrossRef CAS.
- Industrial Dyes: Chemistry, Properties, Applications, ed. K. Hunger, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2003 Search PubMed.
- A. Hakonen, J. E. Beves and N. Stromberg, Analyst, 2014, 139, 3524 RSC.
- A. R. Smith, ACM SIGGRAPH Computer Graphics, 1978, 12, 12 CrossRef.
- K. Cantrell, M. M. Erenas, I. de Orbe-Payà and L. F. Capitán-Vallvey, Anal. Chem., 2010, 82, 531 CrossRef CAS PubMed.
- M. M. Erenas, O. Piñeiro, M. C. Pegalajar, M. P. Cuellar, I. de Orbe-Payá and L. F. Capitán-Vallvey, Anal. Chim. Acta, 2011, 694, 128 CrossRef CAS PubMed.
- M. Ariza-Avidad, A. Nieto, A. Salinas-Castillo, L. F. Capitán-Vallvey, G. M. Miskelly and M. J. Sailor, Nanoscale Res. Lett., 2014, 9, 410 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: (1) Conversion from RGB to HSV colour space; (2) spectral centre of gravity (CG) calculation; (3) preparation of BBS/LLDPE films; (4) preparation, spectroscopic characterization and imaging of the stretched BBS/LLDPE films; and (5) confocal fluorescence microscopy. See DOI: 10.1039/c6cc08360a |
| This journal is © The Royal Society of Chemistry 2017 |