Open Access Article
Open Access ArticleDevelopment of kartogenin-conjugated chitosan–hyaluronic acid hydrogel for nucleus pulposus regeneration
Yanxia
Zhu
 *a,
Jie
Tan
b,
Hongxia
Zhu
c,
Guangyao
Lin
a,
Fei
Yin
a,
Liang
Wang
a,
Kedong
Song
d,
Yiwei
Wang
e,
Guangqian
Zhou
a and
Weihong
Yi
*b
*a,
Jie
Tan
b,
Hongxia
Zhu
c,
Guangyao
Lin
a,
Fei
Yin
a,
Liang
Wang
a,
Kedong
Song
d,
Yiwei
Wang
e,
Guangqian
Zhou
a and
Weihong
Yi
*b
aShenzhen Key Laboratory for Anti-ageing and Regenerative Medicine, Health Science Center, Shenzhen University, Shenzhen 518060, China. E-mail: yanxiazhu@szu.edu.cn; Fax: +86-755-86671906; Tel: +86-755-86671903
bDepartment of Spinal Surgery, Shenzhen Sixth People's Hospital (Nanshan Hospital), Shenzhen, 518060, China. E-mail: szyiwh@163.com; Fax: +86-755-86671906; Tel: +86-755-86671903
cDepartment of Spinal Surgery, Xiaogan Maternity&Child Healthcare Hospital, Xiaogan, 432100, China
dState Key Laboratory of Fine Chemicals, Dalian R&D Center for Stem Cell and Tissue Engineering, Dalian University of Technology, Dalian 116024, China
eBurns Research Group, ANZAC Research Institute, University of Sydney, Concord, NSW 2139, Australia
First published on 6th March 2017
Abstract
Injectable constructs for in vivo gelation have many advantages in the regeneration of degenerated nucleus pulposus. In this study, an injectable hydrogel consisting of chitosan (CS) and hyaluronic acid (HA) crosslinked with glycerol phosphate (GP) at different proportions (CS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GP
GP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HA, 6
HA, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5
1, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 4
2, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 3
3, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 2
4, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, V
6, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V
V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) was developed and employed as a delivery system for kartogenin (KGN), a biocompound that can activate chondrocytes. In vitro gelation time, morphologies, swelling, weight loss, compressive modulus and cumulative release of KGN in hydrogels were studied. For biocompatibility assessments, human adipose-derived stem cells (ADSCs) were encapsulated in these hydrogels. The effects of KGN on stem cell proliferation and differentiation into nucleus pulposus-like cells were examined. The hydrogels with higher concentrations of HA showed a slightly shorter gelation time, higher water uptake, faster weight loss and faster KGN release compared to the hydrogels with lower concentrations of HA. As the KGN-conjugated hydrogel prepared with the proportions 5
V) was developed and employed as a delivery system for kartogenin (KGN), a biocompound that can activate chondrocytes. In vitro gelation time, morphologies, swelling, weight loss, compressive modulus and cumulative release of KGN in hydrogels were studied. For biocompatibility assessments, human adipose-derived stem cells (ADSCs) were encapsulated in these hydrogels. The effects of KGN on stem cell proliferation and differentiation into nucleus pulposus-like cells were examined. The hydrogels with higher concentrations of HA showed a slightly shorter gelation time, higher water uptake, faster weight loss and faster KGN release compared to the hydrogels with lower concentrations of HA. As the KGN-conjugated hydrogel prepared with the proportions 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 displayed good mechanical properties, it was chosen as the optimal gel to promote cell proliferation and differentiation. No significant difference was seen in the expression levels of nucleus pulposus markers induced by KGN or TGF-β. Additionally, inclusion of KGN and TGF-β together did not produce a synergistic effect in inducing nucleus pulposus properties. In conclusion, we have developed a KGN-conjugated CS/HA hydrogel (5
2 displayed good mechanical properties, it was chosen as the optimal gel to promote cell proliferation and differentiation. No significant difference was seen in the expression levels of nucleus pulposus markers induced by KGN or TGF-β. Additionally, inclusion of KGN and TGF-β together did not produce a synergistic effect in inducing nucleus pulposus properties. In conclusion, we have developed a KGN-conjugated CS/HA hydrogel (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) with sustained release of KGN in hydrogel that can promote ADSC proliferation and nucleus pulposus differentiation. This kind of hydrogel may be a simple and effective candidate for the repair of degenerative NP tissue after minimally invasive surgery.
2) with sustained release of KGN in hydrogel that can promote ADSC proliferation and nucleus pulposus differentiation. This kind of hydrogel may be a simple and effective candidate for the repair of degenerative NP tissue after minimally invasive surgery.
Introduction
Degenerative disc disease is the main cause of chronic low back pain and disability in the elderly. As discs degenerate, there is a decrease in the water content and a reduction in type II collagen and proteoglycans in the nucleus pulposus (NP), resulting in structural destruction and flattening of the disc.1,2 Conventional approaches such as conservative treatment and surgical techniques can relieve the patients’ clinical symptoms to some extent.2,3 However, interest in developing biomaterials to regenerate the NP is growing dramatically, as biomaterials (with or without graft cells) provide a new strategy for restoring native tissue structures and the mechanical function. When the diseased NP tissue has been surgically removed, scaffolds constructed with biomaterials can be used for cell- and factor-delivery, which is aimed at tissue regeneration and finally, rehabilitation of normal disc function.Although in vitro cell based engineered tissue has shown promising results in clinical studies, there are some limitations in clinical application, such as invasive surgery, inflammation, and subsequent infection. Injectable in situ-forming hydrogels can thus overcome these limitations, as they merely involve delivery via syringe injection during minimally invasive surgery, introducing the aqueous solution into the body at target sites to fill irregularly shaped defects.4,5In situ-forming hydrogels are particularly suitable for disc transplantation because of their cavity structures, and have become increasingly attractive in NP and cartilage tissue engineering6,7 as well as drug delivery.8
Scaffold materials for cell- and factor-delivery should be biomimetic and should contain components of the extracellular matrix (ECM) in order to illicit specific cellular responses and direct new tissue formation.9 Among various biomaterials, sodium hyaluronate/hyaluronic acid (HA) is a natural, biocompatible and biodegradable polysaccharide.10 Moreover, it is a major component of synovial fluid as well as glycosaminoglycans (GAGs) that are found in the NP and articular cartilage. HA has been used broadly for osteoarthritis treatment,11 as an intra-articular injective material, and has been proven to support cell proliferation and maintain the chondrogenic phenotype.12 It has been demonstrated that HA-based hydrogels can direct recovery or replacement of the endogenous NP for NP tissue engineering and cellular therapies.13 Another suitable candidate for cartilage and NP tissue repair is chitosan. Chitosan is structurally analogous to GAGs,14 and is also non-toxic, water soluble, biodegradable, biocompatible and displays anti-bacterial properties. Chitosan has been investigated extensively for drug delivery systems.15 The chitosan–gelatin scaffold prepared by the freeze-gelation method provides better conditions for NP cell proliferation.16 The advantage of ECM molecules is that it allows cells to maintain their differentiated phenotype for specific tissues.
In addition to the material in the scaffold, growth factors are also important for tissue regeneration. The small molecule KGN can promote the selective differentiation of mesenchymal stem cells (MSCs) into chondrocytes, and has been identified as a chondrogenic and chondroprotective agent.17,18 In a mouse model of osteoarthritis, intra-articular injection of KGN has been demonstrated to reduce tibial plateau cartilage degeneration.19 Accordingly, KGN is expected to be a potential novel therapeutic drug for the treatment of osteoarthritis.
In this study, we constructed a biocompatible CS/HA hydrogel, which has similar mechanical properties to native NP tissue. In addition, we have synthesized a KGN-conjugated CS/HA hydrogel and have demonstrated that sustained release of KGN in the hydrogel can promote adipose-derived stem cell (ADSC) proliferation and NP differentiation, and thus enhance the construction of engineered NP tissue.
Materials and methods
Preparation and fabrication of CS/HA hydrogels
For hydrogel preparation, a batch size of 5 mL each was prepared at ambient temperature. A 2% chitosan (CS, deacetylation 90%, Sigma) stock was prepared in 0.1 M hydrochloric acid, and a 10% β-glycerophosphate (GP, Sigma) stock and 1% sodium hyaluronate (HA, 350 kDa, Huaxi Fureida) stock was prepared by dissolving in distilled water. All stock solutions were left at rest at 4 °C overnight to remove bubbles. The 2% CS, 10% GP and 1% HA solutions were subsequently mixed at different proportions (V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V
V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V, 6
V, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5
1, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 4
2, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 3
3, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 2
4, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) and maintained in a 37 °C water bath prior to use. All percentages in the formulations refer to % (w/v).
6) and maintained in a 37 °C water bath prior to use. All percentages in the formulations refer to % (w/v).
Hydrogel characterization
The pH values of the mixtures were measured using a Seven2Go pH-meter with a viscotrode (Mettler Toledo). Three measurements were taken in series on the same sample; four samples in each group were measured.The interior morphology of the hydrogels was observed using scanning electron microscopy (SEM). Prior to SEM analysis, the samples were dehydrated, dried and gold coated with a sputter coater at 20 mA under 70 mTorr for 1 minute. The surface and cross-sectional morphologies were viewed using a JCM-6000 SEM (JEOL), and pore-size distributions of hydrogels were determined by evaluating a set of at least three SEM images using the linear intercept method.
To observe incorporation of HA in CS hydrogels, the cross-linked CS/HA hydrogels were stained with alcian blue. Briefly, the hydrogels were immersed in 0.5% w/v alcian blue solution dissolved in 10% acetic acid aqueous solution. After staining with gentle shaking for 4 hours, the gels were sequentially washed with 2% acetic acid solution and PBS.
To examine the swelling properties, 1 mL of each hydrogel was weighed before immersing in 5 mL of PBS and maintained at 37 °C for 12 hours. The hydrogels were then removed and immediately weighed with a microbalance after excess water on the surface was absorbed with filter paper. The swelling ratio (SR) was calculated using the following equation: SR = (Ws − Wd)/Wd, where Ws and Wd are the weights of the hydrogels at the swelling state and at the dry state, respectively.
To test the mechanical properties, mixtures of the solutions described above were injected into a 96-well culture plate over 15 minutes to obtain columned hydrogels, and these were cut to the same dimensions (∼6 mm diameter, ∼2 mm height). The Young's modulus was measured in the elastic region of the hydrogels using a Nanotensile testing system (T150 UTM, Agilent) with unconfined compression, up to 20% strain at room temperature. Three measurements were performed per gel and three parallel samples were used.
To examine biodegradability in vitro, the hydrogels were incubated in 3 mL of an enzyme solution (100 U mL−1 hyaluronidase and 10 mg mL−1 lysozyme) in a 37 °C water bath. In brief, hydrogels were pre-weighed (W0) before quickly freezing at −80 °C and lyophilizing at −50 °C. The weight loss of dry hydrogels was monitored as a function of incubation time in PBS or the enzyme solution at 37 °C. At specified time intervals, hydrogels were quickly frozen at −80 °C, lyophilized and weighed (Wt). The weight loss ratio was calculated as 100% × (W0 − Wt)/W0. The weight remaining ratio was defined as 1–100% × (W0 − Wt)/W0.
In vitro release study
For preparation of factor-loaded CS/HA hydrogels, KGN was diluted in 10% GP and mixed homogeneously with the CS/HA solution to a final concentration of 50 μM KGN. To examine the release kinetics of KGN from CS/HA hydrogels, each lyophilized KGN-loaded hydrogel was placed in a well of a 24-well microplate and covered with 1 mL PBS. The total volume of PBS was collected and replaced with the same volume of PBS at each sampling time. The amount of KGN released from each CS/HA hydrogel was evaluated using the HPLC (Ultimate 3000, Dionex) spectrum. In vitro release was measured in five replicates under physiological conditions (pH 7.3, 37 °C, humidified atmosphere) at different time-points (day 1, 2, 3, 4, 5, 6, 7, 8, 10, 14 and 16).Cell proliferation and cytotoxicity
GFP-SD rats were purchased from Guangdong Medical Lab Animal Center. Animals were maintained in accordance with the guidelines of the Manipulative Technique for the Care and Use of Laboratory Animals, China, and approved by the Animal Ethical and Welfare Committee of Shenzhen University (SYXK: 2014-0140). ADSC-GFP was isolated from GFP-SD rats with our improved method.20 ADSCs were cultured and suspended in CS/HA solution at a concentration of 1 × 106 cells per mL. The cross-linked hydrogel was cultured in 1 mL of culture medium (DMEM with 10% FBS) at 37 °C and 5% CO2 in humidified incubators for 14 days. The viability of encapsulated cells was observed under a SEM, to assess cell distribution and cryosections were stained with hematoxylin and eosin (H&E).Based on data obtained from preceding experiments, the 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CS/HA hydrogel was chosen for cell proliferation and differentiation. Cell/hydrogel constructs were washed once with PBS and dead cell nuclei were stained with propidium iodide (PI, Invitrogen) at 37 °C for 30 minutes, and observed using a fluorescence microscope (Leica Microsystems). Proliferation of ADSCs in the gels was measured using the cell counting kit (cck-8, Biosource). Cell/hydrogel constructs were washed once with PBS and incubated with cck-8 solution for 3 hours at 37 °C. The cck-8 fluorescence was assayed at 535 nm (excitation) and 600 nm (emission), with four parallel samples being tested.
2 CS/HA hydrogel was chosen for cell proliferation and differentiation. Cell/hydrogel constructs were washed once with PBS and dead cell nuclei were stained with propidium iodide (PI, Invitrogen) at 37 °C for 30 minutes, and observed using a fluorescence microscope (Leica Microsystems). Proliferation of ADSCs in the gels was measured using the cell counting kit (cck-8, Biosource). Cell/hydrogel constructs were washed once with PBS and incubated with cck-8 solution for 3 hours at 37 °C. The cck-8 fluorescence was assayed at 535 nm (excitation) and 600 nm (emission), with four parallel samples being tested.
Differentiation of ADSCs in hydrogels
Four groups were included in the differentiation study; the first group was composed of KGN-loaded hydrogels encapsulating ADSCs that were cultured with differentiation medium (high glucose DMEM supplemented with 10 ng mL−1 transforming growth factor-β 3 (TGF-β 3), all from Life Technologies). In the second group, the ADSC encapsulating un-loaded hydrogels were cultured with differentiation medium. The third group was composed of KGN-loaded hydrogels encapsulating ADSCs that were cultured with standard culture medium. The fourth control group with non-loaded hydrogels encapsulating ADSCs was cultured with standard culture medium. ADSCs at passage 5 were encapsulated at a concentration of 1 × 106 cells per mL hydrogel. Each experiment was conducted with three replicates, and culture duration was 28 days. Briefly, pellets of ADSCs were resuspended in 1 mL mixed solution to form the hydrogels. The cell-containing hydrogel solutions were dispensed into 24-well tissue culture plates (1 mL per well) and allowed to form gels at 37 °C. NP differentiation was examined by histological analysis and biochemical quantification.NP differentiation analysis
For histological analysis, hydrogel samples from the four experimental groups were rinsed with PBS and embedded in the Tissue-Tek® (OCT) compound, snap frozen in liquid nitrogen and stored at −20 °C. Twenty micrometre serial sections from frozen samples were mounted on Superfrost-Plus microscope slides and dried for 24 hours at room temperature. After fixation with methanol/acetone (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
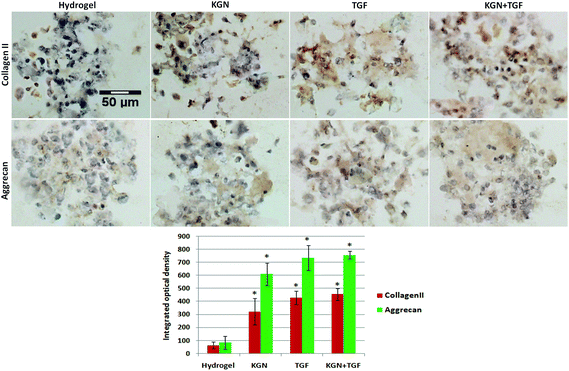
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the cells were rinsed with PBS three times and blocked in 10% normal goat serum for 20 minutes. Samples were incubated with primary antibodies (Anti-Collagen II, Anti-Aggrecan, Abcom) overnight at 4 °C, then rinsed with PBS three times and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Invitrogen) for 2 hours at room temperature. The cells were rinsed with PBS and developed using a DAB kit (Thermo, USA). For semi-quantitative analysis, six pictures for each slide were collected. Image Pro Plus was used to catch the brown area as the area of interest to examine the integrated optical density.
1), the cells were rinsed with PBS three times and blocked in 10% normal goat serum for 20 minutes. Samples were incubated with primary antibodies (Anti-Collagen II, Anti-Aggrecan, Abcom) overnight at 4 °C, then rinsed with PBS three times and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Invitrogen) for 2 hours at room temperature. The cells were rinsed with PBS and developed using a DAB kit (Thermo, USA). For semi-quantitative analysis, six pictures for each slide were collected. Image Pro Plus was used to catch the brown area as the area of interest to examine the integrated optical density.
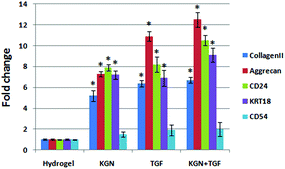
Total RNA from the differentiated cells was obtained using Trizol (Invitrogen). The RNA was reverse transcribed to complementary DNA (cDNA) using the First Strand cDNA kit (Takara) following the manufacturer's protocol. Quantitative polymerase chain reaction (qPCR) analysis was then performed using the Quantitect SYBR Green PCR Master Mix (Takara). Standard curves were generated, and quantities of each transcript were normalized to β-actin as an internal control.
Statistical analysis
Statistical analyses were performed using analysis of variance (ANOVA) followed by the Tukey's post hoc test or Student's t-test. A value of P < 0.05 was considered significant. Results are presented as mean ± standard deviation.Results
Preparation and characterization of hydrogels
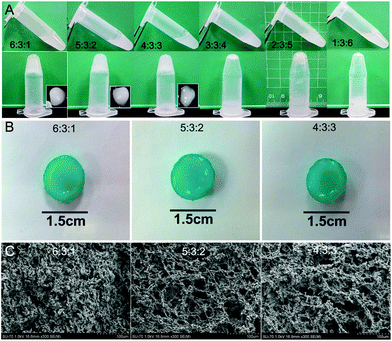
The gelation time of hydrogels was investigated using a vial tilting method (Fig. 1A). The CS/GP/HA solution at 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 showed a sol–gel phase transition after 8 minutes at 37 °C. The gelation time increased from 8 to 14 minutes with an increase in the HA concentration. The gelation time of CS/GP/HA hydrogels further increased to 30 minutes as the proportion of the hydrogel changed to 4
1 showed a sol–gel phase transition after 8 minutes at 37 °C. The gelation time increased from 8 to 14 minutes with an increase in the HA concentration. The gelation time of CS/GP/HA hydrogels further increased to 30 minutes as the proportion of the hydrogel changed to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Table 1). The addition of HA significantly increased the gelation time, however, the CS/GP/HA solution at 3
3 (Table 1). The addition of HA significantly increased the gelation time, however, the CS/GP/HA solution at 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 2
4, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 1
5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 did not change to the gel phase. The pH values of all hydrogel preparations were close to neutral (Table 1).
6 did not change to the gel phase. The pH values of all hydrogel preparations were close to neutral (Table 1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1
2%CS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10%GP 10%GP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1%HA 1%HA |
Time to gel (min) | PH value | Pore size (μm) | Swelling ratio (%) | Young's modulus (MPa) |
|---|---|---|---|---|---|
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 ± 0.5 | 6.8 ± 0.08 | 10–40 | 21 ± 2.5 | 2.9 ± 0.21 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
14 ± 0.6 | 6.92 ± 0.06 | 40–80 | 28 ± 3.2 | 1.6 ± 0.28 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
30 ± 0.6 | 7.03 ± 0.04 | 40–100 | 34 ± 3* | 0.9 ± 0.18* |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
No hydrogel | 7.1 ± 0.07 | — | — | — |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
No hydrogel | 7.17 ± 0.1 | — | — | — |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
No hydrogel | 7.21 ± 0.05 | — | — | — |
CS/HA ratios significantly influenced the swelling ratio of hydrogels in PBS. The equilibrium-swelling ratio of CS/HA with 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 in PBS was 34%, which was significantly higher than the 6
3 in PBS was 34%, which was significantly higher than the 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hydrogel (Table 1). The equilibrium-swelling ratio increased with the proportion of HA in the hydrogels. The values remained stable up to 7 days in PBS.
1 hydrogel (Table 1). The equilibrium-swelling ratio increased with the proportion of HA in the hydrogels. The values remained stable up to 7 days in PBS.
The compressive modulus of the hydrogels was determined by a static mechanical analysis method. The 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 5
1 and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 hydrogels had a significantly higher compressive modulus (2.9 and 1.6 MPa, respectively) than the 4
2 hydrogels had a significantly higher compressive modulus (2.9 and 1.6 MPa, respectively) than the 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 hydrogel (Table 1, p < 0.05). The Young's modulus of fresh NP from humans has been reported to be on average 2 MPa, which is close to that of the 5
3 hydrogel (Table 1, p < 0.05). The Young's modulus of fresh NP from humans has been reported to be on average 2 MPa, which is close to that of the 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 hydrogel. With the incorporation of KGN, the compressive modulus of the composite hydrogels increased, however it was not significantly different from hydrogel only (data not shown).
2 hydrogel. With the incorporation of KGN, the compressive modulus of the composite hydrogels increased, however it was not significantly different from hydrogel only (data not shown).
The CS/HA composite hydrogels without cells were stained with alcian blue to observe HA incorporation and their stability over time (Fig. 1B). The CS/HA hydrogels displayed positive alcian blue staining, indicating the presence of HA in the gels after cross-linking.
The microstructural morphology of dehydrated hydrogels was examined under a SEM (Fig. 1C). Based on the cross-sectional morphology, both hydrogels displayed a continuous and porous structure due to the drying procedure. The pore diameter of the 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CS/HA hydrogel ranged from 10–40 μm, compared to the 5
1 CS/HA hydrogel ranged from 10–40 μm, compared to the 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CS/HA hydrogel with pore diameters of 40–80 μm and the 4
2 CS/HA hydrogel with pore diameters of 40–80 μm and the 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 CS/HA hydrogel with pore diameters ranging from 40–100 μm (Fig. 1C). This difference in the pore size indicates that a higher proportion of CS results in the formation of smaller pore diameters and thus a tighter network structure in thermosensitive hydrogels.
3 CS/HA hydrogel with pore diameters ranging from 40–100 μm (Fig. 1C). This difference in the pore size indicates that a higher proportion of CS results in the formation of smaller pore diameters and thus a tighter network structure in thermosensitive hydrogels.
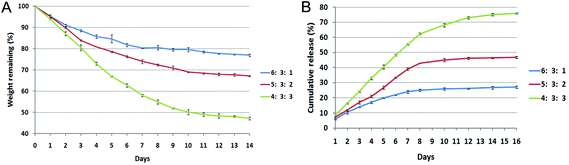
The degradation properties of the composite hydrogels were monitored as a function of incubation time in PBS at 37 °C (Fig. 2A). The ratio of CS/HA had a significant influence on the weight loss behavior of the composite hydrogels. The hydrogels with a higher ratio of CS demonstrated a slower weight loss than the hydrogels with a lower CS composition. Compared with 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 4
2 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 hydrogels, the 6
3 hydrogels, the 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hydrogel formed a more compact hydrogel and thus displayed a steady rate of weight loss for up to 14 days and showed a significantly slower weight loss rate than the other hydrogels. Based on these results, the ratio of 5
1 hydrogel formed a more compact hydrogel and thus displayed a steady rate of weight loss for up to 14 days and showed a significantly slower weight loss rate than the other hydrogels. Based on these results, the ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CS/HA is appropriate for KGN loading and release.
2 CS/HA is appropriate for KGN loading and release.
The in vitro release of KGN from the hydrogels was determined over 16 days (cumulative release shown in Fig. 2B). The 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 CS/HA hydrogels released significantly greater amounts of KGN compared to the 5
3 CS/HA hydrogels released significantly greater amounts of KGN compared to the 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 hydrogels during examination time. In addition, the 6
2 hydrogels during examination time. In addition, the 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hydrogels displayed slow release of KGN from hydrogels, consistent with their degradation properties. The 5
1 hydrogels displayed slow release of KGN from hydrogels, consistent with their degradation properties. The 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CS/HA hydrogel showed a sustained KGN release over the examination period.
2 CS/HA hydrogel showed a sustained KGN release over the examination period.
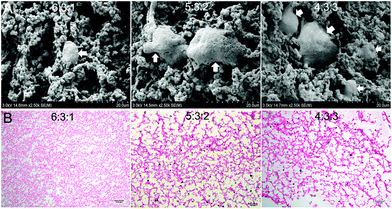
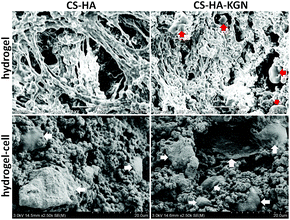
The SEM images of the encapsulated ADSC/hydrogel matrices are presented in Fig. 3A. The residing cells within hydrogels possessed normal spherical or fibroblast-like morphology. CS/HA hydrogels with 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 4
2 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 showed more cell survival and bioactivity.
3 showed more cell survival and bioactivity.
The analysis of the cell-encapsulating hydrogel by H&E staining revealed a relatively uniform distribution of cells throughout the scaffold. CS/HA hydrogels with 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 4
2 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 showed more cells than that of 6
3 showed more cells than that of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was consistent with the observations under a SEM. However, hydrogels with 4
1, which was consistent with the observations under a SEM. However, hydrogels with 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 were too fragile to handle during examination.
3 were too fragile to handle during examination.
Cell viability and proliferation inside the hydrogel
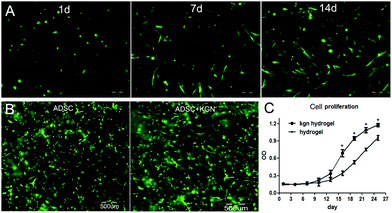
To assess the suitability of the CS/HA hydrogel to support cell survival, ADSCs were encapsulated in CS/HA hydrogels and cell viability was assessed by propidium iodide (PI) staining of cell-hydrogel constructs over two weeks in culture (Fig. 4B). Most of the cells encapsulated inside CS/HA hydrogels were viable, and maintained a round morphology after one day in culture. CS/HA hydrogels maintained a good overall viability of ADSCs for 14 days in culture, and some cells that were dispersed in the hydrogel showed fibroblast-like morphology (Fig. 4A). After PI staining, the live/dead encapsulated ADSCs within hydrogels after 14 days in culture were imaged by fluorescence microscopy (Fig. 4B). Round or fibroblast-like ADSCs were uniformly distributed in both CS/HA and KGN-conjugated hydrogels. Most of the encapsulated ADSCs survived in copolymer hydrogels after 14 days in culture. Observations using SEM were consistent with the results seen with PI staining (Fig. 5), however, the encapsulation process and two-dimensional culture may still result in cell death. A higher number of live cells were observed in the KGN-conjugated hydrogels than in the pure hydrogels after 14 days of incubation, which was confirmed using the cell proliferation cck-8 assay. In addition, small particles with KGN were observed inside or on the surface of the hydrogels (Fig. 5). Proliferation of encapsulated ADSCs in the CS/HA hydrogel was monitored in culture media at different time-points over 26 days by cck-8 (Fig. 4C). The cells showed significantly higher metabolic activity at days 14–26 compared to days 0–7 (P < 0.001), indicating that the cells could grow and proliferate within CS/HA hydrogels.NP differentiation of the encapsulated ADSCs
The NP induced cell-hydrogel constructs were examined by immunohistological methods to identify the distribution of collagen and aggrecan that are generated. A non-induced construct (control) showed less or minimal staining for collagen II and aggrecan in culture medium at day 28 (Fig. 6). Constructs induced with either KGN or TGF-β expressed collagen II and aggrecan to similar degrees, and induction with both factors together did not further promote the differentiation of ADSCs compared to KGN or TGF-β alone. All the differentiation groups had significantly higher expression of collagen II and aggrecan compared to that of the non-induced groups. The quantitative results from real-time PCR were consistent with the immunohistological staining results (Fig. 7).Discussion
The cell density within the NP decreases with aging, which results in the loss of proteoglycan synthesis and a decline in the production of important ECM proteins such as aggrecans and type II collagen.21 Chitosan is structurally analogous to GAGs, and HA is a major component of the ECM in the NP.10,12,14 Hydroxyl groups in HA can cross-link with amino groups in CS. CS when combined with glycerol phosphate and HA can undergo a sol–gel transition at 37 °C by covalent cross-linking.22 We have shown in this study that increasing the HA concentration in the CS/HA thermo-sensitive hydrogel increases the gelation time, which may be attributable to the presence of more hydrophilic groups among HA chains.In addition to gel thermosensitivity, other important requirements include high water content, biodegradability as well as mechanical properties,23 which are important to the mechanical function of the disc after implantation, and it is important that the matrix secreted from implanted cells is able to replace the hydrogel over time after the implantation. The hydrogels with a higher HA content displayed a loose structure, consequently increasing the exposure of hydrophilic polymer chains to water molecules at 37 °C, leading to enhanced water absorption and significantly faster weight loss. This is likely due to the complicated entanglement of macromolecular chains,5 and accordingly, a higher ratio of CS resulted in a tighter network structure and a smaller pore diameter in the hydrogels.
We chose the hydrogel that demonstrated similar mechanical properties to native NP tissue. Since the microstructure, mechanical properties and high water content of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 hydrogels are very similar to those of the extracellular matrix of natural NP tissue, these hydrogels may provide an environment for maintaining cell bioactivity and preserving the cell phenotype. We used ADSCs for the regeneration of engineered NP tissue, because they can be easily obtained from autologous adipose tissue, and we have demonstrated that their proliferation and differentiation ability are much stronger than that of bone marrow derived stem cells. These cells have been widely investigated for use in cartilage and other tissue regeneration.24–26 ADSCs showed good morphology and strong proliferation ability in CS/HA hydrogels. Incorporation of ADSCs into the CS/HA hydrogel may aid ADSC proliferation and NP differentiation since native NP cells prefer to live in a three dimensional microenvironment. This also enables mechanical load transduction, which is important for the synthesis of the NP matrix.27 Incorporating cells into the CS/HA hydrogel solution reduces clustering and poor distribution of transplanted cells. Moreover, after gelation, the hydrogel provides a temporary three-dimensional matrix to increase cell retention and survival.
2 hydrogels are very similar to those of the extracellular matrix of natural NP tissue, these hydrogels may provide an environment for maintaining cell bioactivity and preserving the cell phenotype. We used ADSCs for the regeneration of engineered NP tissue, because they can be easily obtained from autologous adipose tissue, and we have demonstrated that their proliferation and differentiation ability are much stronger than that of bone marrow derived stem cells. These cells have been widely investigated for use in cartilage and other tissue regeneration.24–26 ADSCs showed good morphology and strong proliferation ability in CS/HA hydrogels. Incorporation of ADSCs into the CS/HA hydrogel may aid ADSC proliferation and NP differentiation since native NP cells prefer to live in a three dimensional microenvironment. This also enables mechanical load transduction, which is important for the synthesis of the NP matrix.27 Incorporating cells into the CS/HA hydrogel solution reduces clustering and poor distribution of transplanted cells. Moreover, after gelation, the hydrogel provides a temporary three-dimensional matrix to increase cell retention and survival.
The CS/HA hydrogel can also create a favorable NP-like microenvironment due to the incorporation of ECM components present in NP tissue.28–32 The presence of CS, which is analogous to GAGs and the ECM component HA may support the growth and deposition of cells, which may play a special role in modulating NP differentiation and function.6,13
CS/HA hydrogels can also serve as a delivery device not only for mobilizing stem cells to the injection site, but also for sustainable release of bioactive molecules or growth factors. KGN is a recently characterized molecule that promotes the differentiation of stem cells into chondrocytes for cartilage regeneration. KGN is a low molecular weight and hydrophobic compound, in which the amino groups of chitosan can couple covalently to its carboxyl groups.18 We conjugated KGN into the CS/HA hydrogel to enhance the aqueous solubility and sustained release from the hydrogel. Similar to previous studies,18 the conjugation of KGN to the hydrogel enhanced the proliferation and differentiation of NP cells. During differentiation of stem cells, KGN frees core-binding factor (CBF)-b, which may bind to the DNA-binding transcription factor RUNX1 to activate the transcription of collagen II and aggrecan.17 In addition, KGN has similar ability to induce differentiation to TGF-β, but cannot further promote or enhance the effect of TGF-β, therefore is a suitable replacement of TGF-β for NP and cartilage differentiation and regeneration. After construction of NP-like tissues, the next step is implantation. As we all know, the inflammation environment is one of the key factors for DDD, CD54 can be used as a biomarker to evaluate the inflammation-associated disc degeneration, because the expression of CD54 was insignificant in younger NP tissue, and showed stronger expression in aged NP tissue.33 There is no significant increase of CD54 expression after differentiation in the hydrogel, which indicated that there is no inflammation reaction occurring in our constructed hydrogel, and will cause no harm to native disc after implantation.
Conclusions
In our study, chitosan was chosen to mimic the GAG structure in the NP ECM, and HA as a major component of NP ECM, to construct a CS/HA injectable hydrogel that can support proliferation of ADSCs and promote differentiation towards NP-like tissues. In addition, a KGN-conjugated hydrogel has the potential to support ADSC distribution and related factor delivery, which significantly promotes NP cell differentiation in hydrogels and development of engineered NP tissues. Thus, this type of hydrogel with encapsulated ADSCs and KGN may fill damaged NP defects and can be applied during minimally invasive surgery. However, further work is needed to understand the mechanism of how the CS/HA hydrogel affects the production of type II collagen and aggrecan by ADSCs, and animal studies will be needed to investigate the efficacy of NP regeneration of the CS/HA hydrogel–ADSC system.Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81301597) and the Shenzhen Special funds for the Emerging Strategic Industry Development (No. JCYJ20150525092940984, JCYJ20150525092940973, JCYJ20160422090807181).Notes and references
- D. S. Mern, A. Beierfuß, C. Thomé and A. A. Hegewald, J. Tissue Eng. Regener. Med., 2014, 8, 925–936 CrossRef CAS PubMed.
- D. Drazin, J. Rosner, P. Avalos and F. Acosta, Adv. Orthop., 2012, 2012, 1–8 CrossRef PubMed.
- D. Oehme, T. Goldschlager, J. V. Rosenfeld, P. Ghosh and G. Jenkin, Neurosurg. Rev., 2015, 38, 429–445 CrossRef PubMed.
- S. H. Söntjens, D. L. Nettles, M. A. Carnahan, L. A. Setton and M. W. Grinstaff, Biomacromolecules, 2006, 7, 310–316 CrossRef PubMed.
- H. Tan, J. P. Rubin and K. G. Marra, Organogenesis, 2010, 6, 173–180 CrossRef PubMed.
- M. B. Nair, G. Baranwal, P. Vijayan, K. S. Keyan and R. Jayakumar, Colloids Surf., B, 2015, 136, 84–92 CrossRef CAS PubMed.
- Y. P. Singh, N. Bhardwaj and B. B. Mandal, ACS Appl. Mater. Interfaces, 2016, 8, 21236–21249 CAS.
- S. Atta, S. Khaliq, A. Islam, I. Javeria, T. Jamil, M. M. Athar, M. I. Shafiq and A. Ghaffar, Int. J. Biol. Macromol., 2015, 80, 240–245 CrossRef CAS PubMed.
- S. Ravindran, Q. Gao, M. Kotecha, R. L. Magin, S. Karol, A. Bedran-Russo and A. George, Tissue Eng., Part A, 2012, 18, 295–309 CrossRef CAS PubMed.
- H. Tan, C. M. Ramirez, N. Miljkovic, H. Li, J. P. Rubin and K. G. Marra, Biomaterials, 2009, 30, 6844–6853 CrossRef CAS PubMed.
- F. Wang and X. He, Exp. Ther. Med., 2015, 9, 493–500 CAS.
- C. W. Ha, Y. B. Park, J. Y. Chung and Y. G. Park, Stem Cells Transl. Med., 2015, 4, 1044–1051 CrossRef CAS PubMed.
- D. H. Kim, J. T. Martin, D. M. Elliott, L. J. Smith and R. L. Mauck, Acta Biomater., 2015, 12, 21–29 CrossRef CAS PubMed.
- H. Naderi-Meshkin, K. Andreas, M. M. Matin, M. Sittinger, H. R. Bidkhori, N. Ahmadiankia, A. R. Bahrami and J. Ringe, Cell Biol. Int., 2014, 38, 72–84 CrossRef CAS PubMed.
- Y. Shi, Z. Xiong, X. Lu, X. Yan, X. Cai and W. Xue, J. Mater. Sci. Mater. Med., 2016, 27, 169 CrossRef PubMed.
- Z. Karimi, M. Ghorbani, B. Hashemibeni and H. Bahramian, Adv. Biomed. Res., 2015, 4, 251 CrossRef PubMed.
- K. Johnson, S. Zhu, M. S. Tremblay, J. N. Payette, J. Wang, L. C. Bouchez, S. Meeusen, A. Althage, C. Y. Cho, X. Wu and P. G. Schultz, Science, 2012, 336, 717–721 CrossRef CAS PubMed.
- M. L. Kang, J. Y. Ko, J. E. Kim and G. I. Im, Biomaterials, 2014, 35, 9984–9994 CrossRef CAS PubMed.
- Y. Ono, S. Ishizuka, C. B. Knudson and W. Knudson, Cartilage, 2014, 5, 172–180 CrossRef CAS PubMed.
- Y. Zhu, T. Liu, K. Song, R. Ning, X. Ma and Z. Cui, Mol. Cell. Biochem., 2009, 324, 117–129 CrossRef CAS PubMed.
- C. K. Kepler, D. G. Anderson, C. Tannoury and R. K. Ponnappan, J. Am. Acad. Orthop. Surg., 2011, 19, 543–553 CrossRef PubMed.
- S. Kaderli, C. Boulocher, E. Pillet, D. Watrelot-Virieux, A. L. Rougemont, T. Roger, E. Viguier, R. Gurny, L. Scapozza and O. Jordan, Int. J. Pharm., 2015, 483, 158–168 CrossRef CAS PubMed.
- S. Xue, D. Pei, W. Jiang, Y. Mu and X. Wan, Polymer, 2016, 99, 340–348 CrossRef CAS.
- Y. Zhu, T. Liu, K. Song, X. Fan, X. Ma and Z. Cui, Cell Biochem. Funct., 2008, 26, 664–675 CrossRef CAS PubMed.
- Y. Zhu, T. Liu, H. Ye, K. Song, X. Ma and Z. Cui, Stem Cells Dev., 2010, 19, 1547–1556 CrossRef CAS PubMed.
- R. Kasir, V. N. Vernekar and C. T. Laurencin, Regen. Eng. Transl. Med., 2015, 1, 42–49 CrossRef PubMed.
- A. T. Francisco, R. J. Mancino, R. D. Bowles, J. M. Brunger, D. M. Tainter, Y. T. Chen, W. J. Richardson, F. Guilak and L. A. Setton, Biomaterials, 2013, 34, 7381–7388 CrossRef CAS PubMed.
- H. Park, B. Choi, J. Hu and M. Lee, Acta Biomater., 2013, 9, 4779–4786 CrossRef CAS PubMed.
- C. R. Correia, L. S. Moreira-Teixeira, L. Moroni, R. L. Reis, C. A. van Blitterswijk and M. Karperien, et al. , Tissue Eng., Part C, 2011, 17, 717–730 CrossRef CAS PubMed.
- K. L. Spiller, S. A. Maher and A. M. Lowman, Tissue Eng., Part B, 2011, 17, 281–299 CrossRef CAS PubMed.
- Y. C. Huang, V. Y. Leung, W. W. Lu and K. D. Luk, Spine J., 2013, 13, 352–362 CrossRef PubMed.
- Y. C. Huang, J. P. Urban and K. D. Luk, Nat. Rev. Rheumatol., 2014, 10, 561–566 CrossRef PubMed.
- X. Tang, L. Jing, W. J. Richardson, R. E. Isaacs, R. D. Fitch, C. R. Brown, M. M. Erickson, L. A. Setton and J. Chen, Identifying molecular phenotype of nucleus pulposus cells in human intervertebral disc with aging and degeneration, J. Orthop. Res., 2016, 34, 1316–1326 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |