X-ray fluorescence (XRF) analysis of porcelain: Background paper
Analytical Methods Committee AMCTB No. 77
First published on 12th April 2017
Abstract
This technical brief outlines how X-ray fluorescence (XRF) can be used for the investigation of cultural heritage objects made of porcelain. It discusses the types of questions that can be answered with XRF and provides an overview of how the method works.
X-ray fluorescence (XRF) is a non-destructive analytical method that allows the identification and quantification of the chemical elements present in the surface of a wide variety of objects. It is currently widely applied in the investigation and characterisation of art objects as well as geological and archaeological materials. Because XRF is non-destructive, the method is particularly suitable for the analysis of complete objects, such as porcelain vessels, when sampling is not an option because it would affect the integrity of the item. In the last 20 years, portable instruments have become commercially available, allowing samples or objects to be analysed in situ (see AMC Technical Brief No. 41). Most XRF instruments are capable of detecting the majority of elements in the periodic table, ranging from magnesium to uranium. This makes XRF an ideal tool to characterise the materials used in the manufacture of porcelain bodies, glazes, enamel decoration and gilding.
Types of porcelain
There are two main types of porcelain, defined by chemical composition and firing temperature: hard paste and soft paste.Hard paste ‘true’ porcelain is a hard white ceramic that was first produced in China and later in Japan and Europe. It is made from kaolinite clay and petuntse (a rock comprising mica, feldspar and quartz).
Soft paste porcelain is a less translucent, slightly porous white ceramic that began to be manufactured in Europe in the 18th century, although there were attempts at production in the preceding centuries. It is made from white clay mixed with a glass frit (sand, gypsum, soda, salt, alum and nitre) fused together with chalk and lime.
Bone china is a porcelaneous body that was developed in Britain in the 18th century and is considered to be a type of soft paste. It is composed of kaolin/ball clay and Cornish stone mixed with a bone ash flux. Both soft paste and bone china wares were first produced in imitation of the ‘true’ hard paste porcelain as the hard paste formula and raw materials were not widely known at the time.
Porcelain is typically coated with a silica-rich glaze, which has a chemical composition that is compatible with the paste. For example, hard paste glazes consist of silica with an alkali such as lime or potash and soft paste glazes are based on silica with lead oxide. A range of decorative components can be applied, including underglaze, in-glaze, on-glaze (enamel) and gilded decoration. Porcelain colours are made from pigments, such as oxides of copper, cobalt and iron, which produce a range of shades depending on the firing conditions and type of glaze to which they are applied.
Applications of XRF analysis
XRF is used on porcelain and its decorative components in order to help answer questions on authenticity, provenance, date and restoration. The raw materials and recipes employed by different production centres result in unique chemical compositions that can be determined by using XRF. Raw materials often changed over time, possibly owing to technological or economic considerations. Each time a new recipe was used, the composition would be subtly different. An example of one of these changes involved the use of green enamels at the Meissen factory in Saxony. In the 18th century, green enamels were coloured using copper oxide but, at the beginning of the 19th century, chromium oxide green was introduced. Thus the presence of chromium green is a good marker for 19th century decoration. Temporal compositional differences form the basis of scientific dating for most types of porcelain. In order for this dating to be effective, results must be compared with databases compiled from the analysis of established materials, or new reference objects must be analysed.X-ray fluorescence
XRF is a method that can determine concentrations of major, minor and, in some cases, trace elements by using X-rays. The most common XRF set-up used in heritage laboratories is energy dispersive (ED) XRF. During an ED-XRF experiment, a high-energy X-ray beam hits the surface of the object or sample under observation. This in turn emits X-rays of lower energy, a behaviour known as ‘fluorescence’. Each chemical element produces fluorescent X-rays with energies that are unique to that element. The emitted X-rays are displayed as a spectrum with peaks of varying heights at different energies. The energy of a peak identifies the chemical element producing it and the height of a peak can usually be linked to the abundance of that element in the sample. Software is then used to measure and quantify the abundance of detected elements. Reference materials are always used to calibrate the procedure or to check for bias in the results.Equipment and methods
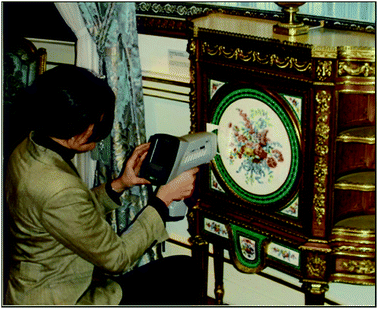
The three most common instruments used in heritage laboratories are (Fig. 1 and 2):• Benchtop analysers,
• Mobile open architecture instruments for use in museum galleries, and
• Handheld analysers.
Benchtop XRF instruments have a large chamber in which the object for analysis is placed. These instruments have an adjustable beam diameter, usually between 0.2 and 3 mm, which means that fine areas of paint and gilding can be analysed. Some benchtop instruments can map elemental distributions spatially across the surface of the object, allowing variations in the pigments to be viewed visually (Fig. 3).
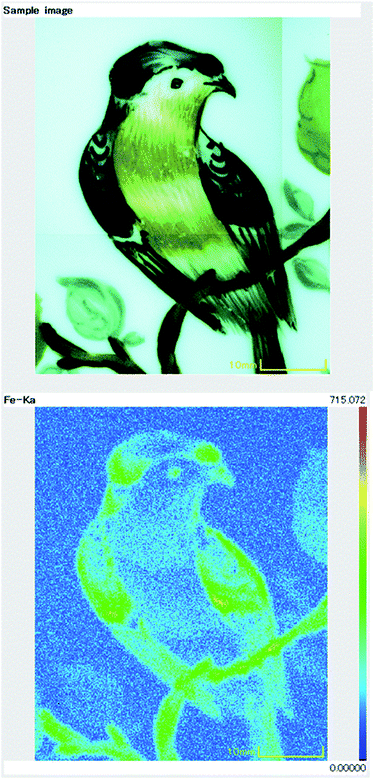 | ||
| Fig. 3 Visual appearance (top) and XRF map (bottom) of iron distribution across the surface of an enamelled porcelain plate. | ||
Mobile models have comparable beam sizes (0.2 to 1.5 mm), but have the added benefit of being able to work on large immoveable objects in situ. Benchtop and mobile devices are operated in combination with software on a connected computer.
Handheld devices are fully portable, weighing only a few kilograms, which means that they can be easily carried into museums, private collections and auction houses, and that valuable objects no longer need to be transported to the laboratory. The beam size is larger and usually non-adjustable on handheld devices, with beam sizes ranging from 3 mm to 8 mm. Handheld devices are operated via software on the back of the instrument or by connecting them to laptop computers. As handheld and mobile instruments have open X-ray beams, the instruments must be used within a controlled area and the operator has to abide by strict health and safety regulations.
In order to conduct an analysis, the whole object is placed either in the chamber (for benchtop instruments), or directly in front of the instrument head within 1 cm of the beam on the mobile or handheld device. Sampling is not required and the method is completely non-destructive. Handheld devices usually have to touch the object to reduce radiation scatter. Measurement times typically range from 30 to 100 seconds. Longer measuring times are required for the detection of ‘light’ elements (those of low atomic number such as sulfur, aluminium and silicon) and trace elements (those at concentrations lower than roughly 0.1% by mass). Shorter measuring times are often employed for handheld devices as it can be difficult for the operator to hold the XRF in position for long periods.
Results and interpretation
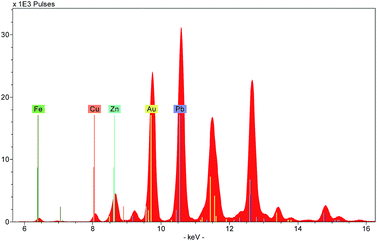
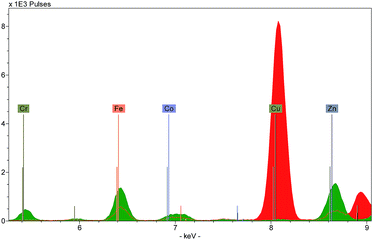
Three types of output can be generated by XRF instruments: an X-ray spectrum, a list of spectrum peak areas, and relative concentrations calculated via calibration data. The raw output is displayed as a spectrum, with each peak uniquely representing a particular chemical element (Fig. 4). Each peak is identified with the originating element by using software installed on the instrument or on a connected computer. The height of each peak is related to the abundance of the element. Several spectra can be overlaid on the screen allowing the user to discern visually the differences between the ranges and abundances of elements in different targets.Because of this, spectra provide a useful means to characterise a material quickly. For example, the colouring agent in a green enamel may be identified as copper or chromium (Fig. 5), or a glaze may be characterised as leaded or non-leaded by the presence or absence of high lead peaks. The characterisation of the basic materials can be useful for cataloguing purposes, documenting the history of technology, assessment of the condition of an object, identifying restorations and repairs, and dating.
Quantitative analysis
Some types of XRF software can calculate and compare the size of the elemental peaks on the spectrum: this is called ‘peak area analysis’ and allows the user to determine the relative proportions of elements present. The data can be downloaded and used in statistical software packages. Peak area analysis is used to find simple patterns in the data that may indicate the use of different recipes or sources of raw materials. For example, at the Meissen factory, early 18th century porcelain pastes and glazes were produced by using a calcium sulfate flux that was later augmented and replaced by a potassium feldspar flux. These flux materials may be identified using peak area analysis by examining the ratio of calcium to potassium (Fig. 6). As these different fluxes were used at different dates, this type of information can be used as an aid to dating.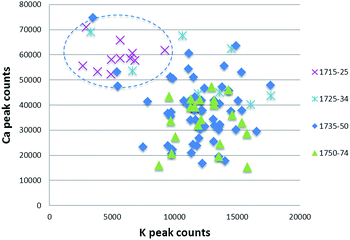 | ||
| Fig. 6 Scatter plot of Meissen glazes produced in 1715–74, highlighting the high calcium/low potassium composition of early glazes. | ||
A more general type of analytical result comprises determining concentrations of the elements (as contrasted with elemental ratios). These are calculated from peak areas but in conjunction with information from reference materials containing known concentrations of the elements. Some XRF instruments have in-built theoretical ‘fundamental parameter’ (FP) programs to calculate percentage data, based on both the measured peak intensities and the fundamental physics specific to that instrument and the elements concerned. Results are normalised to 100% and are material-specific. FP programs are calibrated by using a specific set of elements, so if an object under test has a different suite of elements the normalised quantitative results will be inaccurate. The accuracy of these programs must therefore be checked with a reference material similar to the test object.
The widely-recommended way to generate accurate percentage data is to conduct an ‘empirical calibration’ whereby the operator calibrates the instrument using a set of reference materials with known compositions similar to the test material. A second (disjoint) set of reference materials is then analysed to check the accuracy of the calibration. The calibration can then be used to determine the composition of the porcelain under analysis.
Care must be taken in attempting to analyse thin layers of light material as the X-ray beam can penetrate into the substrate material and the results may represent a mixture of one or more layers. Analysis depth is typically in the region of 40 μm (micrometres) for a high lead glaze or 500 μm for a lime-alkali glaze. Heterogeneity of the material will reduce the reliability of the results, and therefore several readings should be taken from nearby areas and the results averaged.
In order that useful interpretations may be made, results must be compared against a database of established materials or by conducting XRF analyses of comparable reference objects. Published databases can be found in academic journals such as Archaeometry, Journal of Archaeological Science, X-Ray Spectrometry and Journal of the American Ceramic Society.
Useful literature
A. Bezur and F. Casadio, The analysis of porcelain using handheld and portable X-ray fluorescence spectrometers, in Handheld XRF for Art and Archaeology, Studies in Archaeological Sciences 3, ed. A. N. Shugar and J. L. Mass, Leuven, Leuven University Press, 2012.F. Casadio, A. Bezur, K. Domoney, K. Eremin, L. Lee, J. L. Mass, A. Shortland and N. Zumbulyadis, X-ray fluorescence applied to overglaze enamel decoration on eighteenth- and nineteenth-century porcelain from central Europe, Stud. Conserv., 2012, 57, 61–72.
K. Domoney, A. Shortland and S. Kuhn, Characterization of 18th-Century Meissen Porcelain using SEM-EDS, Archaeometry, 2012, 54(3), 454–474.
W. Kingery, The Development of European Porcelain, High Technology Ceramics: Past, Present and Future. Ceramics and Civilization III., The American Ceramic Society, Westerville, 1986, pp. 153–179.
X-Ray Fluorescence Spectrometry (XRF) in Geoarchaeology, Springer, New York, ed. M. Shackley, 2011.
P. J. Potts and M. West, Portable X-ray fluorescence analysis, AMC Technical Briefs No. 41, 2009.
Portable X-ray Fluorescence Spectrometry: Capabilities for In Situ Analysis, ed. P. Potts and M. West, Royal Society of Chemistry, Cambridge, 2008.
K. Yu, Attribution of antique Chinese blue-and-white porcelains using Energy Dispersive X-Ray Fluorescence (EDXRF), in Radiation in Art and Archeometry, ed. D. Creagh and D. Bradley, Elsevier Science, Amsterdam, 2000, pp. 317–345.
Kelly Domoney (Cranfield University and Ashmolean Museum, University of Oxford)
This Technical Brief was prepared by the Heritage Science Subcommittee and approved by the Analytical Methods Committee on 29/12/16.
| This journal is © The Royal Society of Chemistry 2017 |