Open Access Article
Open Access ArticleSpiegelmers as potential receptors for cTnI diagnostics†
Zsuzsanna
Szeitner
 a,
Anna
Doleschall
a,
Anna
Doleschall
 a,
Marina
Varga
a,
Marina
Varga
 b,
Katalin
Keltai
b,
Katalin
Keltai
 c,
Katalin
Révész
c,
Katalin
Révész
 c,
Róbert E.
Gyurcsányi
c,
Róbert E.
Gyurcsányi
 d and
Tamás
Mészáros
d and
Tamás
Mészáros
 *ae
*ae
aDepartment of Medical Chemistry, Molecular Biology and Pathobiochemistry, Semmelweis University, Budapest, Hungary
bDepartment of Transplantation and Surgery, Semmelweis University, Budapest, Hungary
cDepartment of 3rd Internal Medicine, Semmelweis University, Budapest, Hungary
dMTA-BME “Lendület” Chemical Nanosensors Research Group, Department of Inorganic and Analytical Chemistry, Budapest University of Technology and Economics, Budapest, Hungary
eMTA-BME Research Group for Technical Analytical Chemistry, Budapest University of Technology and Economics, Budapest, Hungary. E-mail: meszaros.tamas@med.semmelweis-univ.hu; Fax: +36 1 266 3802; Tel: +36 1 266 2615
First published on 28th July 2017
Abstract
We demonstrate the first application of a nuclease resistant aptamer, the so-called Spiegelmer, in a sandwich-type affinity assay by quantitative assessment of cardiac Troponin I (cTnI) in blood serum samples. To this end, we used a bead-based homogenous proximity assay (AlphaLISA) in which luminescence is generated by the Spiegelmer and antibody-modified donor and acceptor beads brought into proximity by their binding to cTnI.
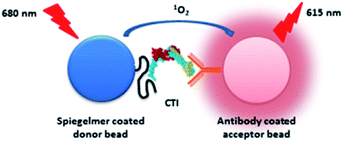
Prompt, on-site diagnosis of Acute Coronary Syndrome (ACS) is the prerequisite for timely triage of patients with acute chest pain. Two subunits of the cardiac troponin complex, troponin I (cTnI) and troponin T (cTnT), are derived from genes specifically expressed in the myocardium; therefore, their appearance in the systemic circulation is a highly selective indicator of cardiomyocyte necrosis.1,2 Presently, all clinically applied diagnostic devices of cardiac troponins are based on immunoassays and thus require antibodies.3 The standardization of cTnI immunoassays is complicated by the presence of various forms of cTnI in circulation, i.e., monomer protein, in interaction with cTnT (TI) and as a tertiary troponin complex that includes additionally Troponin C (CTI). Further difficulties are raised by the association of cTnI with autoantibodies and heparin, and by posttranslational modifications.4 It was reported that assays using different antibody pairs may reach up to 40 fold deviations of measured cTnI concentration.5 This problem is addressed by assay-specific threshold levels for each commercial cTnI assay, which are not interchangeable and therefore may lead to misinterpretations.6 Aptamer-based assays may provide a solution for these standardization problems by appropriate epitope targeting and counterselection to critical interferents. Furthermore, aptamers – in contrast to antibodies – can be produced by cost-effective chemical synthesis with orientated modifications and preserve their functionality even under harsh prevailing conditions; thus, they can outperform antibodies in diagnostic applications.7 On the other hand, the most generally examined specimens of human diagnostics, blood plasma and serum, include a panel of nucleases that might expeditiously degrade oligonucleotides severely limiting leveraging aptamers as receptors of biomarker determining assays.8 With these advantages and drawbacks of aptamers in mind, we previously developed a cTnI selective Spiegelmer9 for a specific N-terminal epitope of cTnI with a rationally designed selection procedure. Spiegelmers10 are enantiomeric aptamers, which are composed of L-2′-deoxyribose units and thus fully withstand enzymatic degradation. Hitherto, Spiegelmers have been used for therapeutic purposes with promising results but their diagnostic exploitation has not been explored yet.11 In the present study, we attempted the analytical application of our Spiegelmer to test whether it can substitute antibodies of sandwich type assays for quantitative assessment of cTnI in human serum. To implement these proof-of-principle studies, we used a highly sensitive, bead-based homogenous proximity assay, the so-called AlphaLISA.12 This method relies on selective antibody coated donor and acceptor beads that are brought into proximity through binding to the pertinent analyte. Upon light exposure, the donor bead released singlet oxygen reaches the acceptor beads in vicinity and produces a luminescence signal.13 Here, we used streptavidin donor and protein A acceptor beads of AlphaLISA, which could be conveniently modified using a biotin tagged Spiegelmer and a cTnI selective antibody, respectively (Fig. 1).
Since our previously generated Spiegelmer was directed against an N terminal peptide sequence of cTnI, we chose a commercial antibody that was raised against a C terminal epitope of our protein of interest to avoid the rivalry of the two receptors for the same binding site. To study the potential clinical impact of the selected Spiegelmer, experiments were implemented in serum, a general specimen of ACS diagnosis. Blood serum is one of the most complex proteomes of diagnostics and the concentrations of its protein components vary by up to 10 orders of magnitude. Besides albumin, various antibodies of μmolar range concentrations are the most prevalent proteins of serum.14 In order to attain the optimal signal intensity of AlphaLISA, the protein A acceptor bead has to be incubated with a cTnI selective antibody at low nanomolar concentration. Consequently, the cTnI selective antibody is replaced on the protein A acceptor bead upon addition of serum with inherently high antibody concentration. To overcome this shortcoming, a cTnI selective antibody was covalently linked to the protein A bead by using the dimethyl pimelimidate crosslinking agent prior to its addition to the blood serum. The labelled Spiegelmer and streptavidin donor bead could be infused into the serum without any pretreatment, due to the extraordinarily selective and strong interaction of streptavidin and biotin.
Myocardial injury results in the release of cTnI in various forms. In order to assess the applicability of our Spiegelmer as a synthetic cTnI receptor of sandwich assays, diluted, troponin-free serum was spiked with various amounts of CTI, the generally accepted reference material of cTnI measurment. It is well documented that cTnI is prone to degradation and binding to plastic and glass surfaces.15,16 Therefore, the dilution linearity was analysed by determining the cTnI concentration of spiked specimens with a clinically applied, two-step chemiluminescence immunoassay, which is distributed by ARCHITECT under the brand name STAT Troponin-I. In the first step, cTnI binds to antibody-coated paramagnetic microparticles. Following separation and washing of the beads, an anti-troponin-I acridinium-labeled conjugate is added in the second step. After washing and addition of enhancer solutions, the chemiluminescence reaction produced signal is measured. The obtained data illustrated the problems of cTnI standardization. The measured cTnI concentrations of CTI spiked samples were app. two times lower than the calculated ones (ESI Table 1†).
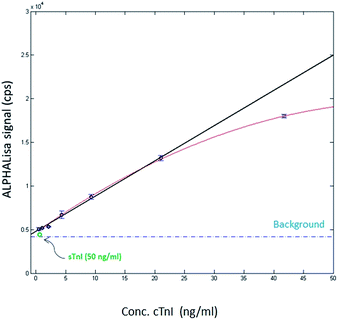
Next, the samples were studied by using AlphaLISA. To this end, a protein A acceptor bead-coupled cTnI selective antibody and biotin labelled Spiegelmer were added to the dilution series and the mixture was incubated for 1 h. In the following step, the mixture was completed with streptavidin donor beads and incubated for a further hour and then the luminescence signals were measured. The obtained data demonstrated low standard deviation of the parallel samples and the plotted luminescence signal intensity showed good linearity in the ng ml−1 range of troponin (Fig. 2).
These results suggest that the Spiegelmer is suitable for cTnI measurement in diluted serum but did not provide information about the specificity of the developed assay. To study whether our AlphaLISA could discriminate the skeletal muscle and cardiomyocyte isoform of troponin I, a cross-reactivity study was implemented by spiking the serum with troponin-free 50 ng ml−1 final assay concentration of sTnI and conducting the AlphaLISA measurement as described above. The produced luminescence signal remained at the level of those detected with cTnI-free serum samples implying high specificity of the Spiegelmer-based sandwich assay (Fig. 2).
The optimal cTnI level monitoring assay is expected to detect the various forms of the protein; therefore, it was also studied if the developed AlphaLISA is capable of measuring the concentration of the cTnI monomer. It was described that the cTnI monomer was even more prone to degradation than the CTI complex.17 Therefore, the actual concentration of the recombinant cTnI supplemented serum samples were also determined by STAT Troponin-I measurement. According to the obtained data, the calculated concentration was app. 20 times higher than the determined one (ESI Table 2†) again indicating the challenges of cTnI measurement. Having determined the concentration of cTnI spiked samples, the AlphaLISA produced luminescence was detected following the previously applied protocol. The intensity of the luminescence signal correlated well with the amount of the added cTnI indicating that the Spiegelmer-antibody sandwich assay is capable for monitoring the cTnI monomer too (Fig. 3).
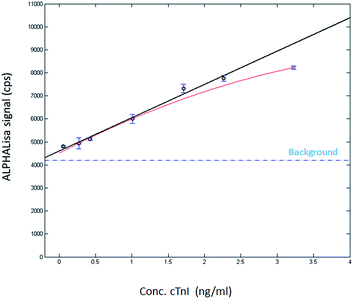 | ||
| Fig. 3 AlphaLISA signal as a function of the concentration of cTnI. The diluted serum was formulated with recombinant cTnI. The adjusted R-square value of the linear response was 0.985. | ||
Conclusions
Presently, most of the diagnostic devices adopt antibodies for selective detection of biomarker proteins. Despite some essential advantages over antibodies, leveraging of aptamers for diagnostics is still awaited. The presented proof-of-principle study shows the first example of using a fully nuclease resistant aptamer, the so-called Spiegelmer, for the development of a cTnI detecting sandwich assay. The obtained results demonstrate that the Spiegelmer can ensure the required selectivity and can detect various forms of cTnI in serum at physiologically relevant low pM concentrations. Although its LOD is much higher than those of the presently available high sensitivity cTnI measuring kits,18 the developed protocol shows applicability of Spiegelmers as receptors of sandwich assays for biomarker analysis of blood serum. The most important features of diagnostic receptors are the selectivity and the KD value of receptor and target protein complexes, since smaller the KD the lower the expected LOD is. An extended analysis has revealed that the monoclonal antibodies of high sensitivity cTnI diagnostic kits possess KD values in the 74 nM to 2.6 μM range, which are at least an order of magnitude higher than that of our Spiegelmer (3.5 nM).9 Therefore, it is expected that further assay optimization or usage of more sensitive detection approaches would make achievable the desired LOD of cTnI assays by employing Spiegelmers. Notwithstanding, the presented results signify the applicability of Spiegelmers as diagnostic receptors; hence, they could pave the way for the development of oligonucleotide-based, biomarker detecting devices.Acknowledgements
The financial support of the VKSZ_14-1-2015-0004 of the National Research, Development and Innovation Office and the Lendület program of the Hungarian Academy of Sciences (LP2013-63/13) is gratefully acknowledged.Notes and references
- J.-P. Jin, Z. Zhang and J. A. Bautista, Crit. Rev. Eukaryotic Gene Expression, 2008, 18, 93–124 CrossRef CAS.
- F. K. Korley and A. S. Jaffe, J. Am. Coll. Cardiol., 2013, 61, 1753–1758 CrossRef PubMed.
- M.-I. Mohammed and M. P. Y. Desmulliez, Lab Chip, 2011, 11, 569–595 RSC.
- A. Katrukha, A. Bereznikova, V. Filatov and T. Esakova, Clin. Chem. Lab. Med., 1999, 37, 1091–1095 CrossRef CAS PubMed.
- R. H. Christenson, S. H. Duh, F. S. Apple, G. S. Bodor, D. M. Bunk, M. Panteghini, M. J. Welch, A. H. B. Wu and S. E. Kahn, Clin. Chem., 2006, 52, 1685–1692 CAS.
- M. Panteghini, Clin. Chem., 2005, 51(5), 803–804 CAS.
- V. Thiviyanathan and D. G. Gorenstein, Proteomics: Clin. Appl., 2012, 6, 563–573 CrossRef CAS PubMed.
- S. Shigdar, J. Macdonald, M. O'Connor, T. Wang, D. Xiang, H. Al Shamaileh, L. Qiao, M. Wei, S.-F. Zhou, Y. Zhu, L. Kong, S. Bhattacharya, C. Li and W. Duan, Sensors, 2013, 13, 13624–13637 CrossRef CAS PubMed.
- Z. Szeitner, G. Lautner, S. K. Nagy, R. E. Gyurcsányi and T. Mészáros, Chem. Commun., 2014, 50, 6801–6804 RSC.
- S. Klussmann, A. Nolte, R. Bald, V. A. Erdmann and J. P. Furste, Nat. Biotechnol., 1996, 14, 1112–1115 CrossRef CAS PubMed.
- A. Vater and S. Klussmann, Drug Discovery Today, 2015, 20, 147–155 CrossRef CAS PubMed.
- E. F. Ullman, H. Kirakossian, A. C. Switchenko, J. Ishkanian, M. Ericson, C. A. Wartchow, M. Pirio, J. Pease, B. R. Irvin, S. Singh, R. Singh, R. Patel, A. Dafforn, D. Davalian, C. Skold, N. Kurn and D. B. Wagner, Clin. Chem., 1996, 42, 1518–1526 CAS.
- G. Lautner, Z. Balogh, A. Gyurkovics, R. E. Gyurcsányi and T. Mészáros, Analyst, 2012, 137, 3929 RSC.
- R. Pieper, C. L. Gatlin, A. J. Makusky, P. S. Russo, C. R. Schatz, S. S. Miller, Q. Su, A. M. McGrath, M. A. Estock, P. P. Parmar, M. Zhao, S.-T. Huang, J. Zhou, F. Wang, R. Esquer-Blasco, N. L. Anderson, J. Taylor and S. Steiner, Proteomics, 2003, 3, 1345–1364 CrossRef CAS PubMed.
- N. A. Morjana, Biotechnol. Appl. Biochem., 1998, 28(2), 105–111 CAS.
- D. C. Gaze and P. O. Collinson, Open Heart, 2014, 1, e000108 CrossRef PubMed.
- A. G. Katrukha, A. V. Bereznikova, V. L. Filatov, T. V. Esakova, O. V. Kolosova, K. Pettersson, T. Lövgren, T. V. Bulargina, I. R. Trifonov, N. A. Gratsiansky, K. Pulkki, L. M. Voipio-Pulkki and N. B. Gusev, Clin. Chem., 1998, 44, 2433–2440 CAS.
- Troponin Assay Analytical Characteristics – IFCC, http://www.ifcc.org/ifcc-scientific-division/documents-of-the-sd/troponinassayanalyticalcharacteristics/.
Footnote |
| † Electronic supplementary information (ESI) available: Reagents and experimental details. See DOI: 10.1039/c7ay01777d |
| This journal is © The Royal Society of Chemistry 2017 |