Open Access Article
Open Access ArticleEnzymatic digestion as a tool for removing proteinaceous templates from molecularly imprinted polymers
Júlia
Erdőssy
a,
Eszter
Kassa
b,
Anita
Farkas
b and
Viola
Horváth
 *c
*c
aMTA-BME “Lendület” Chemical Nanosensors Research Group, Department of Inorganic and Analytical Chemistry, Budapest University of Technology and Economics, Szent Gellért tér 4, Budapest, H-1111, Hungary
bDepartment of Inorganic and Analytical Chemistry, Budapest University of Technology and Economics, Szt. Gellért tér 4, Budapest, H-1111, Hungary
cMTA-BME Research Group of Technical Analytical Chemistry, Szt. Gellért tér 4, Budapest, H-1111, Hungary. E-mail: vhorvath@mail.bme.hu
First published on 3rd July 2017
Abstract
Proteinaceous templates are often immobilized prior to polymerization in molecular imprinting which usually entails the need for digestion as a tool for subsequent template removal. The efficiency of digestion, however, has never been investigated in detail in such a context despite the well-known importance of the template removal step in creating selective binding sites. We have demonstrated that native proteins are often not efficiently cleaved by proteinase K, a highly efficient protease enzyme that can digest even keratin. We have studied and optimized the digestion conditions of a model protein, horseradish peroxidase (HRP), by comparing the obtained fragments to those predicted by in silico digestion. The highest cleaving efficiency was obtained after denaturation of the protein with a surfactant and reduction of its disulphide bridges. The protocol developed with HRP was also tested on avidin and was demonstrated to be applicable for template removal from HRP- or avidin-imprinted polymers.
Introduction
The expanding field of biotechnology, biomarker-based medical diagnostics and targeted drug delivery increasingly relies on the selective recognition of proteins. The gold standard of selective protein capturing is still based on antibodies despite the well-known deficiencies of such biological compounds including high cost, instability and lack of regenerability. Molecularly imprinted polymers (MIPs) offer a promising alternative to overcome these shortcomings. These synthetic adsorbents can be made selective toward a target compound simply by preparing them in the presence of the target which acts as a template during polymer formation and generates its impression in the polymer. These cavities, which are complementary chemically and in shape to the target, can rebind it after the templating molecules have been removed. In this process, template removal is a key step since this will liberate selective binding sites. Incomplete template removal leads to loss of binding capacity and the remaining template might later leach out, falsifying measurements. Macromolecular templates, such as proteins, can be challenging to remove from the polymer network due to their large size and hindered mobility. The protein might even become covalently bound to the polymer during free radical polymerization although this has been excluded experimentally in special cases.1 Intentional immobilization of the template, on the other hand, is often applied to increase the binding site density in thin polymer films or to perform site-directed imprinting for better selectivity.2–4 Weak, reversible bonds might break during polymerization; therefore the formation of stable, covalent bonds is preferred for this purpose. This approach usually entails proteolytic digestion as a means of template removal unless a cleavable linker or a sacrificial support is applied for protein immobilization.5Enzymatic digestion has emerged as a tool for template removal in imprinting strategies using a free, dissolved template, too, since it can be performed under milder conditions than solvent extraction or electromigration techniques6,7 and the obtained peptide fragments are expected to be more easily washed out of the MIP than the intact protein. Therefore proteases with broad cleaving specificity are preferred which are able to break down the protein to very short peptides or even single amino acids, e.g. subtilisin,8 proteinase K,4,6,9–12 pronases13–17 or pepsin.18 Trypsin, which breaks down proteins to relatively large (several kDa) peptide fragments was also applied in some cases but satisfactory rebinding was only obtained if the digestion was followed by extensive washes with a surfactant solution.19,20 When tryptic digestion was compared with surfactant + acetic acid washes as a template removal method it was found that despite the higher template recovery the digested MIP's binding capacity was 4.5 times poorer than that of the washed MIP.21 It was hypothesized that the protein fragments remaining in the digested polymer hinder rebinding. Others, in contrast, suppose that such fragments might even be partly responsible for target recognition.8 Taguchi et al. experimentally proved this concept by creating MIPs in which the template protein was covalently bonded to the polymer at three or five positions, leaving behind three or five peptide fragments after peptic digestion.18 It is noteworthy that peptic fragments are generally smaller than tryptic fragments so it is possible that larger fragments sterically hinder, while small fragments promote the rebinding by offering additional interaction points. This would further underline the importance of choosing a protease with broad cleaving specificity and ensuring the completion of all possible cleavings.
Current protein digestion protocols used in template removal are rather simple. In most cases the MIP is incubated with a buffered solution of the proteolytic enzyme (Table 1). The experimental conditions (enzyme concentration, buffer composition and pH, temperature, and length) are generally not optimized (Table 1). These methods, although efficient in specific cases, are not suitable for the removal of proteins that are more resistant to proteolysis in their native state. Only a few of the current protocols include denaturation of the protein template,6,9,18 despite the fact that the folded or denatured state can influence a protein's susceptibility to proteolytic degradation.24 Moreover, the presence of disulphide bonds25,26 or glycosylation27,28 also contributes to the proteolytic resistance of proteins. Accordingly, high template recoveries were only reported for proteins that do not contain disulphide bonds and hence are easily digested (e.g. haemoglobin,21 cytochrome C,19 maltose binding protein22). The work of Bossi et al.23 seems to be an exception where peroxidases that are difficult to digest were efficiently removed by trypsin. However, this observation was based on the decrease of peroxidatic activity which does not necessarily mean the physical removal of the whole protein only the destruction of the active site.
| Protease | Template | Pre-treatment | Protease concentration and digestion procedure | Recovery (measurement method) | Digestion optimization | Ref |
|---|---|---|---|---|---|---|
| a BSA: bovine serum albumin, CBC: carbonate-bicarbonate buffer, Cyt c: cytochrome c, Hb: haemoglobin, Lys: lysozyme, MBP: maltose binding protein, Myo: myoglobin, OVA: ovalbumin, PB(S): phosphate buffer(ed saline), RT: room temperature, TBS: tris buffered saline. | ||||||
| Pepsin | Cyt c (bonded to the forming polymer) | Wash with 0.5% SDS, 1 M NaCl | 24 h at 37 °C | 18 | ||
| Pronase E | Interferon α2a | 0.4 mg ml−1 in 0.1 M CBC pH 8.5, 24 h | Incubation length (1–24 h): complete after 15 h | 13 | ||
| Pronase E | RNAse, Lys | 0.4 mg ml−1 in 0.1 M CBC pH 8.5, 24 h | ≥62% (fluorescence) | Incubation length (3 or 24 h): 3 h is enough for low protein loadings | 14 | |
| Pronase E | Lys | 0.4 mg ml−1 in PBS pH 7.0, 24 h at RT | 30–40% (fluorescence; depends on template loading) | 15 | ||
| Pronase XXI | BSA, Lys | 0.4 mg ml−1 in 0.1 M PB pH 8, 48 h | 16 | |||
| Pronase XXV | Urease, BSA, Myo, Hb | 0.4 mg ml−1 in 0.1 M PB pH 8.5, overnight or 0.8 mg ml−1 in 0.1 M PB pH 8.0, 2–160 h | 35–75% (scintillation; depends on type of protein and length of digestion) | Incubation length (2–160 h) | 17 | |
| Proteinase K | Trypsin | 1% SDS at 100 °C | 0.1 mg ml−1 in 100 mM NaCl + 50 mM CaCl2, 12 h at 40 °C, digest 2× | 6 | ||
| Proteinase K | Protein A | 0.5 mg ml−1 in PBS, 2.5 h at RT | 11 | |||
| Proteinase K | Ricin toxin chain A (adsorbed on Au) | 0.5% Tween in PBS | 3 mg ml−1 in PBS for 2 h at 37 °C, 0.5% Tween | Incubation length: 2 h is enough | 9 | |
| Proteinase K | Myo (covalently immobilized) | 0.4 mg ml−1 in PBS, overnight in the dark | 4 | |||
| Proteinase K | Myo (adsorbed on Au) | 0.5 mg ml−1 in PBS, overnight in the dark | 12 | |||
| Proteinase K | MBP | 0.4 mg ml−1 in 100 mM NaCl + 50 mM CaCl2, 12 h at 40 °C, in the dark | 98% (fluorescence) | 22 | ||
| Savinase | RNAse | Washing with Savinase | 1 | |||
| Subtilisin A | BSA, fibrinogen (cov. immob.) | 1 mg ml−1, 1 h at RT | 8 | |||
| trypsin | Cyt c (free or cov. immob.) | 0.02 mg ml−1 in TBS pH 8, 1 h, then 1 h in 10% SDS | 100% (Coomassie staining) | 19 | ||
| Trypsin | BHb | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 enzyme 1 enzyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate ratio, 12 h at 40 °C, PBS washing substrate ratio, 12 h at 40 °C, PBS washing |
87.4% (UV-VIS absorption) | Enzyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate ratio (10–30–50 substrate ratio (10–30–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 yielded 58–75–87% recovery) 1 yielded 58–75–87% recovery) |
21 | |
| Trypsin | Peroxidases, Hb | 18 h | >90% (residual peroxidatic activity) | 23 | ||
| Trypsin | OVA (adsorbed on glass) | 3 h at 37 °C, then wash with 2% SDS in 0.4% NaOH (30 min, 60 °C) | 20 | |||
The efficiency of template removal is scarcely given in the literature and the methods used to determine the template recovery are often inadequate. Recoveries are mostly calculated by using fluorescently labelled templates and measuring the fluorescence of the digest.14,15,22 This can overestimate the amount of template removed from the polymer, since unlabelled proteins or protein fragments can still remain in the MIP. The same problem can arise with radiolabelling.17 UV-VIS absorption measurement of the released template21 or stained polymer19 is not sensitive enough.
We therefore aimed at setting up a generally applicable protocol for the removal of protein templates from molecularly imprinted polymers and propose a more reliable method to quantitate template removal.
For this purpose we have selected one of the proteases with a broad cleaving specificity: proteinase K, as it is a single, well-defined enzyme (unlike e.g. pronase, which is a mixture of proteases) and works very efficiently; it can digest even keratin. Proteinase K has already been introduced in the field of molecular imprinting by the group of Sales.4,11,12 We first optimized the digestion conditions on HRP, a model protein dissolved in buffer. We have estimated the template recovery by monitoring the amount of single amino acids in the digest by HPLC-MS-MS which offers a more reliable and sensitive means to quantitate template removal than most current approaches. The optimized procedure was then tested on another protein, avidin in solution, and finally on molecularly imprinted polymers fabricated with covalently immobilized HRP or avidin.
Experimental
Chemicals and materials
RapiGest™ SF was purchased from Waters (Milford, MA, USA), HRP (horseradish peroxidase) was from Roche (Basel, CH), and avidin, 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDC), 3-[(2-aminoethyl) dithio]propionic acid hydrochloride (AEDP) and tris(2-carboxyethyl)phosphine hydrochloride (TCEP) were from Thermo Fischer Scientific (Waltham, MA, USA). Gradient grade acetonitrile, ethanol and 96% acetic acid were from Merck (Darmstadt, DE), and toluene and N,N′-ethylene-bis(acrylamide) (EBis) were from VWR (Radnor, PA, USA). Monodispersed polystyrene beads of 792 nm diameter with surface carboxyl groups (Polybead Carboxylate) were obtained from Polysciences (Warrington, PA, USA). Tetraethyl orthosilicate (TEOS) was obtained from Alfa Aesar, 25% ammonia solution from Riedel de Haen (Seelze, DE), and (3-amino-propyl)triethoxysilane (APTES) from Roth (Karlsruhe, DE). (11-mercaptoundecyl)tetra(ethylene glycol) (HS-TEG), 4-cyano-4(phenylcarbonothioylthio)pentanoic acid (CTA), ammonium persulfate (APS), N,N,N′,N′-tetramethylene-diamine (TEMED), iodoacetamide (IAM), DL-dithiothreitol (DTT), 3,4-ethylenedioxythiophene (EDOT), poly(sodium 4-styrenesulfonate) (NaPSS, average MW ∼ 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), N-isopropylacrylamide and an amino acid standard mix containing each L-amino acid in 2.5 nM concentration in 0.1 N HCl were from Sigma Aldrich (St. Louis, MO, USA).
000), N-isopropylacrylamide and an amino acid standard mix containing each L-amino acid in 2.5 nM concentration in 0.1 N HCl were from Sigma Aldrich (St. Louis, MO, USA).
Thin MIP film preparation for avidin recognition
A molecularly imprinted polymer film was prepared on a 10 MHz gold-coated quartz crystal based on a previously described method.5 Briefly, 6.25 mg of 0.792 μm diameter carboxylated polystyrene latex beads were suspended in 85 μl 10 mM phosphate buffer pH 5 (PB) and activated with 10 μl EDC (200 mg ml−1) for 15 minutes. Next, AEDP (450 μl, 5 mg ml−1) was added to the activated beads and allowed to react for 60 min. The beads were then collected by centrifugation (10 min, 12000g) and were washed 5 times with PB, and finally resuspended in PB to give a concentration of 29 mg ml−1. The carboxyl group of the AEDP moiety was activated with 10 μl 200 mg ml−1 EDC for 15 min with end-over-end mixing, followed by diluting the suspension ten times with PBS pH 7.4 and adding 2.5 mg avidin to the beads. After 60 min mixing the beads were collected by centrifugation (10 min, 12000g) and were washed twice with PBS and thrice with ultrapure water, and finally resuspended in ultrapure water to give a concentration of 2.5 mg ml−1.64 μl of the bead suspension was drop-cast onto the surface of a gold electrode (0.205 cm2) on a 10 MHz AT-cut quartz crystal (Gamry Instruments, Warminster, PA, USA), pre-treated for 15 min in a UV/ozone cleaner (Novascan PSD Pro UV Ozone System), and slowly dried at a controlled relative humidity of 75% (T = 23 °C). PEDOT/PSS was then potentiostatically deposited in the voids of the particle array at 0.9 V (vs. Ag/AgCl/3 M NaCl, counter electrode: Pt) from an aqueous solution of 10 mM EDOT and 25 mM NaPSS. The amount of charge passed during polymer deposition was set to embed the beads up to half their height (8.06 mC). The crystal was then mounted in a cell, and subjected to the optimized digestion procedure.
As a control template removal process, another MIP-coated crystal was treated with 50 mM TCEP for 30 min followed by rinsing with 0.05% Tween20 and water, in order to remove the protein from the beads.
In both cases, the crystal was dried then immersed in toluene to dissolve the polystyrene beads. The bare gold surfaces exposed upon this step were finally blocked with 0.1 mM HS-TEG in water for 60 min.
The modification of the beads with avidin was confirmed and quantitated using the Micro BCA protein assay kit (Thermo Fischer Scientific Inc., Waltham, MA, USA).
QCM measurement
The MIP-coated quartz crystal was mounted in a cell (ALS Co. Ltd, Tokyo, Japan) and connected to a Gamry eQCM 10M electrochemical quartz crystal microbalance. After obtaining a baseline in 500 μl PBT (10 mM phosphate buffer pH 7.0 containing 0.01% Tween20) binding of avidin was measured by replacing 50 μl portions of the solution with avidin stock solutions of increasing concentration and recording the stabilized frequency after each step.Core-shell MIP preparation for HRP recognition
First, silica core particles were synthesized by the Stöber method.29,30 Briefly, 10 ml TEOS, 133 ml ethanol, 5.667 ml 25% ammonia solution and 1.575 ml water were mixed and stirred for 24 h. The obtained particles were collected by centrifugation at 2934 rcf for 60 min, washed four times with ethanol and dried under vacuum at room temperature. Second, surface amino groups were introduced by reacting 0.67 g silica particles, dispersed in 12 ml anhydrous toluene, with 0.24 ml APTES for 15 h under stirring. The particles were washed 3 times with acetone and dried under vacuum at room temperature. In a third step CTA was immobilized to the aminated silica particles by EDC coupling. To this end, 25 mg NH2-functionalized silica particle was mixed with 64 μmol CTA and 128 μmol EDC in 1.25 ml 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v% acetonitrile
20 v/v% acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water and was agitated for 2 hours at room temperature. The particles were washed with acetonitrile three times and resuspended in 0.5 ml acetonitrile. In the fourth step, 158 nmol (6.912 mg) periodate activated HRP and 369 μmol sodium cyanoborohydride in 7 ml PBS pH 7.4 were added to 48 mg CTA-functionalized silica particle and gently agitated for 1 hour at room temperature. The particles were then washed with PBS 5 times. The fifth step consisted of polymerizing a molecularly imprinted shell on the particles by mixing 47 mg HRP-conjugated silica particle with 42.9 mg N-isopropyacrylamide, 3.4 mg EBis, 3.17 mg APS and 2.05 μl TEMED in 9.2 ml water. After 72 h at 45 °C the particles were washed with water 5 times. Finally, the particles were subjected to the optimized digestion procedure.
water and was agitated for 2 hours at room temperature. The particles were washed with acetonitrile three times and resuspended in 0.5 ml acetonitrile. In the fourth step, 158 nmol (6.912 mg) periodate activated HRP and 369 μmol sodium cyanoborohydride in 7 ml PBS pH 7.4 were added to 48 mg CTA-functionalized silica particle and gently agitated for 1 hour at room temperature. The particles were then washed with PBS 5 times. The fifth step consisted of polymerizing a molecularly imprinted shell on the particles by mixing 47 mg HRP-conjugated silica particle with 42.9 mg N-isopropyacrylamide, 3.4 mg EBis, 3.17 mg APS and 2.05 μl TEMED in 9.2 ml water. After 72 h at 45 °C the particles were washed with water 5 times. Finally, the particles were subjected to the optimized digestion procedure.
HRP rebinding test
The obtained core–shell particles were equilibrated for 1 h with HRP solutions of different concentration in a 2500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 phase ratio after which the HRP concentration in the supernatant was determined by enzyme assay using a tetramethyl benzidine (TMB) liquid substrate system from Sigma Aldrich. A 50 μl HRP-containing sample was incubated at room temperature with 200 μl TMB solution for 30 min, followed by the addition of 50 μl 1 M sulfuric acid and reading the absorbance at 450 nm.
1 phase ratio after which the HRP concentration in the supernatant was determined by enzyme assay using a tetramethyl benzidine (TMB) liquid substrate system from Sigma Aldrich. A 50 μl HRP-containing sample was incubated at room temperature with 200 μl TMB solution for 30 min, followed by the addition of 50 μl 1 M sulfuric acid and reading the absorbance at 450 nm.
Digestion procedure
In the optimized in-solution digestion procedure 10 μl 0.2% RapiGest™ SF surfactant was added to 25 μl of 1 mg ml−1 protein in 10 mM ammonium acetate buffer pH 8. Next, 3 μl 100 mM DTT was added and the sample was shaken at 250 rpm for 60 min. Afterwards, 27.5 μl CaCl2 (final concentration: 4.3 mM) and 4.7 μl 200 mM IAM were added and the sample was kept in the dark for 45 min. Finally, 25 μl 1 mg ml−1 proteinase K was added and the digestion was allowed to proceed at 37 °C with gentle shaking for 24 h. The reaction was stopped by adding 3 μl glacial acetic acid and shaking the mixture at 37 °C for 30 min. The sample was then centrifuged for 10 min at 12100g and the supernatant was analysed by HPLC-MS-MS.Digestion of the polymer-embedded template proteins was performed in the following manner. The Av-MIP coated quartz crystal was mounted in a cell and was wetted with 25 μl 10 mM ammonium acetate buffer pH 8, while in the case of the HRP-MIP 3 mg core–shell particles were suspended in 25 μl 10 mM ammonium acetate buffer pH 8. To both samples, 10 μl 0.2% RapiGest™ SF was added, followed by the steps in the in-solution digestion protocol. After 24 h of proteolysis the supernatant was removed from the polymers and acidified with 3 μl glacial acetic acid. After shaking at 37 °C for 30 min it was centrifuged for 10 min at 12100g and the supernatant was analysed by HPLC-MS-MS. The Av-MIP was finally rinsed with 0.05% Tween20 and water, while the HRP-MIP was washed with a methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 1
water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture containing 1% acetic acid in order to remove the protein fragments from the polymers.
1 mixture containing 1% acetic acid in order to remove the protein fragments from the polymers.
HPLC-MS-MS analysis
A Perkin Elmer Series 200 HPLC system was used for amino acid analysis. 10 μl of the digest was injected on a SeQuant ZIC-pHILIC column (Merck, 150 × 2.1 mm, 5 μm, 200 Å polymeric beads) with a 20 × 2.1 mm guard column. The autosampler was set to 10 °C. Gradient elution at a flow rate of 0.1 ml min−1 was performed with acetonitrile (eluent A) and 20 mM ammonium acetate buffer pH 4 (eluent B): equilibration with initial conditions at 33% B, increase to 80% B from 0 to 12 min, reversion to the starting conditions (33% B) from 12 to 12.5 min, re-equilibration with the initial composition from 12.5 to 30 min (total run time: 30 min).The HPLC system was interfaced with an AB Sciex 4000 QTRAP mass spectrometer (Applied Biosystems, Framingham, MA, USA). The positive electrospray ionization parameters were as follows: curtain gas: 35, collision gas: medium, ionspray voltage: 5500, temperature: 350, nebulizer and drying gas: 50 and 40, entrance potential: 11. The selected amino acids were quantified in the multiple reaction monitoring (MRM) mode, and the settings for each monitored transition are listed in Table 2. Analysis and data acquisition were performed using Analyst software, version 1.4.2 (AB SCIEX, Framingham, MA, USA).
| Amino acid | MRM transition (m/z) | DP | CE | CXP |
|---|---|---|---|---|
| a DP: declustering potential, CE: collision energy, CXP: collision cell exit potential. | ||||
| Valine | 118 → 72 | 40 | 20 | 4 |
| Threonine | 120 → 103 | 70 | 25 | 10 |
| (Iso)leucine | 132 → 86 | 45 | 15 | 8 |
| Phenylalanine | 166 → 120 | 35 | 25 | 10 |
Results and discussion
Optimizing the digestion protocol with HRP
Although digestion has been applied in several cases as a tool for template removal from molecularly imprinted polymers, its efficiency has never been investigated in detail and the digestion conditions have never been thoroughly optimized. We therefore aimed at filling this gap and proposing a digestion procedure that can be generally applied to remove protein templates from MIPs.We first investigated the digestion of a native protein in solution using proteinase K. We chose HRP as our model protein due to its high stability; its four disulphide bridges and eight glycosylated sites make it a more challenging candidate for digestion. We hypothesized that a procedure able to digest HRP will also be successful on other, less stable proteins. To evaluate the completion of the process we performed an in silico digestion of HRP with proteinase K. PeptideCutter31 predicts the cleavage sites and resulting peptides of a given amino acid sequence using a given protease or chemical. Proteinase K was predicted to perform 148 cleavings on horseradish peroxidase (sequence according to Welinder et al.32) resulting in 1–7 amino acid long peptides. The majority of the single amino acids in the digest are predicted to be leucines (7 mol isoleucine and 15 mol leucine per 1 mol HRP), valine (11 mol), alanine (10 mol), phenylalanine (10 mol) and threonine (9 mol). We have therefore developed an HPLC-MS-MS method to measure the amount of these amino acids in experimental digests and compare them with the predicted values. Alanine was not included in our investigations due to difficulties in its quantitation.
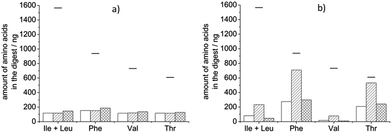
Native HRP was digested by mixing it with proteinase K in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in 10 mM NH4OAc pH 8 buffer and reacting for 5 h at 37 °C. In this digest we did not find significantly more amino acids than in a blank digest (containing only buffer and proteinase K) suggesting that most of the amino acids detected are the result of the enzyme's self-digestion (Fig. 1a). We assumed that the possible cleavage sites are not accessible in the folded, native state of the protein and therefore we tried two approaches to facilitate cleaving: (i) denaturation of the protein with a surfactant, and (ii) reduction and alkylation of the disulphide bridges in the protein. For the first purpose, RapiGest™ SF was applied due to its compatibility with MS analysis: this surfactant decomposes into two products in a low pH environment, one of which can be removed from the digest as a precipitate, and the other is an ionic compound that does not interfere with MS analysis.33 The application of RapiGest™ alone, however, was not effective; the amount of amino acids in the digest did not exceed self-digestion levels (Fig. 1a). Reduction of the disulphide bridges was performed with DTT and the obtained cysteines were stabilized by alkylation with IAM. We have found that disruption of the disulphide bridges made it possible for the proteinase K to access some of the cleavage sites in HRP but the digestion was more complete when the protein was first denatured with the surfactant (Fig. 1b). In the previous examples proteinase K was added to HRP in equimass quantities. Proteases are usually applied in much smaller quantities than the protein to be digested (10–1000
1 ratio in 10 mM NH4OAc pH 8 buffer and reacting for 5 h at 37 °C. In this digest we did not find significantly more amino acids than in a blank digest (containing only buffer and proteinase K) suggesting that most of the amino acids detected are the result of the enzyme's self-digestion (Fig. 1a). We assumed that the possible cleavage sites are not accessible in the folded, native state of the protein and therefore we tried two approaches to facilitate cleaving: (i) denaturation of the protein with a surfactant, and (ii) reduction and alkylation of the disulphide bridges in the protein. For the first purpose, RapiGest™ SF was applied due to its compatibility with MS analysis: this surfactant decomposes into two products in a low pH environment, one of which can be removed from the digest as a precipitate, and the other is an ionic compound that does not interfere with MS analysis.33 The application of RapiGest™ alone, however, was not effective; the amount of amino acids in the digest did not exceed self-digestion levels (Fig. 1a). Reduction of the disulphide bridges was performed with DTT and the obtained cysteines were stabilized by alkylation with IAM. We have found that disruption of the disulphide bridges made it possible for the proteinase K to access some of the cleavage sites in HRP but the digestion was more complete when the protein was first denatured with the surfactant (Fig. 1b). In the previous examples proteinase K was added to HRP in equimass quantities. Proteases are usually applied in much smaller quantities than the protein to be digested (10–1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)34,35 to minimize self-digestion. We therefore performed a digestion on HRP pre-treated with RapiGest™, DTT and IAM using only 10% proteinase K compared to HRP's mass. As expected, the amount of amino acids ascribable to self-digestion was significantly smaller than with 10 times more proteinase K (data not shown) but the amount of amino acids in the HRP's digest also decreased. The net digestion was less complete than with a larger amount of protease (Fig. 1b). In conclusion, the optimal performance is obtained when the protein is unfolded, its disulphide bridges are reduced and alkylated prior to digestion which is performed with an equimass quantity of proteinase K.
1)34,35 to minimize self-digestion. We therefore performed a digestion on HRP pre-treated with RapiGest™, DTT and IAM using only 10% proteinase K compared to HRP's mass. As expected, the amount of amino acids ascribable to self-digestion was significantly smaller than with 10 times more proteinase K (data not shown) but the amount of amino acids in the HRP's digest also decreased. The net digestion was less complete than with a larger amount of protease (Fig. 1b). In conclusion, the optimal performance is obtained when the protein is unfolded, its disulphide bridges are reduced and alkylated prior to digestion which is performed with an equimass quantity of proteinase K.
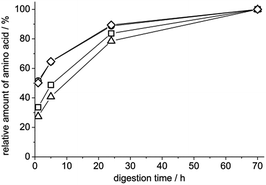
In order to optimize the duration of the proteolysis we performed digestions for different lengths of time. After 24 h the amount of amino acids reached already 78–90% of that obtained after a 70 h digestion which could be considered complete (Fig. 2). Therefore, we have decided to use a 24 h digestion in the final protocol. The amount of single amino acids obtained with this protocol is 10–87% of those predicted by simulation suggesting that not all of the predicted cleavages were performed. It has to be noted, however, that the accuracy of the simulation is limited since it works with quite simple rules and does not take into account any post-translational modifications in the protein, e.g. glycosylation, which could block a cleavage site. On the other hand we believe it is unpractical to further expand the digestion procedure because the obtained amounts of amino acids suggest that the protein is cut into small enough pieces to be easily washed out of a molecularly imprinted polymer.
Testing the digestion protocol on avidin
We further tested the applicability of the optimized digestion procedure on avidin. This protein, found in egg white, consists of four identical polypeptide chains, each of which contain a disulphide bridge and a glycosylated residue. Here again we compared the amount of amino acids found in the digest with those predicted by in silico digestion of avidin. This protein also had to be pre-treated prior to digestion and the combination of denaturation with reduction and alkylation was found to be the most effective. Recoveries were between 7 and 61% for the selected amino acids (Fig. 3).Application of digestion in template removal from MIPs
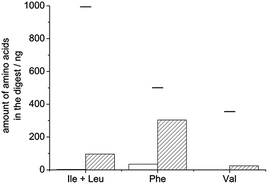
We tested the applicability of digestion as a tool for removing covalently immobilized template proteins from molecularly imprinted polymers in two different systems (in core–shell particles and thin films). In the first case we immobilized HRP on the surface of aminated silica particles. For this purpose we oxidized the vicinal diols in the protein's carbohydrate groups with sodium periodate to aldehydes, which reacted with the amine groups of the silica particles. The obtained Schiff-base was reduced to create a stable covalent bond. A poly(N-isopropylacrylamide-co-bisacrylamide) shell was then allowed to grow around the particles prior to subjecting them to the described digestion procedure. The necessary amount of reagents was determined based on the amount of HRP immobilized on the particles (∼13 μg HRP/mg particle, roughly estimated by enzymatic activity assay). After digestion and washing, the particles retained a weak brown colour indicative of HRP which led us to perform an additional digestion cycle. After the second cycle, the particles were almost colourless. The amount of selected amino acids in the two digests was measured by HPLC-MS-MS and added up. Knowing the approximate amount of the template used for MIP preparation, PeptideCutter predicted the maximum amount of each amino acid that would form if the digestion was complete. Based on the recovery of the different amino acids, 9–133% template removal was calculated. The high variation of recovery is due to the fact that in silico digestion does not reflect experimentally obtained ratios of the monitored amino acids because not all predicted cleavages are actually performed. Fig. 4 shows that the Phe and Thr yields are quite well predicted: the amount of these amino acids found in the experimental digest of dissolved HRP (striped bars) is close to 100% of the values predicted by PeptideCutter, but the predicted cleavages next to valines and leucines are mostly not performed (only 10–15% of the predicted amounts were found from these amino acids). The same trends were observed in the digest of the MIP (Fig. 4, empty bars): values close to the predicted amounts were found from Phe and Thr while much lower recoveries were reached from Val and Ile + Leu. By taking the amounts of amino acids obtained experimentally after solution digestion as the reference instead of the in silico prediction, template recovery from the MIP was between 84 and 147%, indicating that the presence of the polymer matrix did not hinder effective digestion. Note that >100% recoveries can be explained by the fact that the amount of template immobilized onto the silica particles might have been underestimated, because it was measured by the peroxidatic activity of the HRP-coated particles and some activity might have been lost upon immobilization.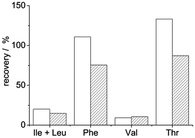 | ||
| Fig. 4 Recoveries (compared to in silico prediction) of selected amino acids found in the digest of MIP-embedded (empty bars) or dissolved (striped bars) HRP. | ||
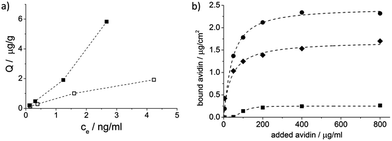
The HRP-MIP particles were also tested for template rebinding and compared with the NIP particles which were prepared identically but without HRP. The higher binding on the MIP indicated successful imprinting (Fig. 5a).
In another example a previously reported imprinting method, based on nanosphere lithography, was adapted.5 Briefly, the template protein was covalently immobilized to polystyrene beads with a cleavable crosslinker, resulting in ∼24 μg avidin/mg bead coverage as estimated by BCA assay. 0.16 mg of the beads was drop-cast to a quartz crystal and embedded up to half their height into a polymer layer which was deposited by electrochemical means. Template removal was then performed either by cleaving the crosslinker to release the protein from the beads (“chemically cleaved” MIP) or by digestion. In both cases the polymer was washed in the end to remove the amino acids or peptide molecules, and finally the beads were dissolved in toluene to obtain the imprinted polymers.
The amount of avidin rebound by the “chemically cleaved” MIP and the proteinase K digested MIP was tested with a quartz crystal microbalance. The digested avidin-MIP could rebind even somewhat greater amounts of avidin than the “chemically cleaved” MIP confirming the suitability of the proposed method for template removal (Fig. 5a). It has to be noted that digestion allows direct immobilization of the template and the cleavable crosslinker was only used for better comparability of the two template removal methods.
These examples show that the proposed digestion method is highly efficient for removing covalently attached protein templates from molecularly imprinted polymers and can be used even for proteins that are difficult to digest.
Conclusions
Until now enzymatic digestion has been used for template removal from protein imprinted polymers only in a few instances and optimal conditions or its efficiency have not been thoroughly investigated. Proteolysis is a prerequisite for washing out covalently immobilized protein templates, an imprinting strategy of great prospect, and can also replace inefficient solvent extraction methods in MIPs prepared with free templates. We have developed a digestion procedure that can be generally applied for template removal in the molecular imprinting of proteins. Here we have demonstrated that native proteins without any pre-treatment are often not efficiently cleaved even by proteinase K, a proteolytic enzyme with broad cleavage specificity. We have developed and optimized a protocol that includes denaturation of the protein with a surfactant followed by reduction of disulphide bonds in order to make it ready for enzymatic digestion. The proposed procedure was tested on two different proteins that are difficult to digest and is expected to be generally suitable. Its applicability to template removal was demonstrated on two different imprinting systems and was found to be effective in removing the protein templates.Acknowledgements
This work was supported by the Hungarian Scientific Research Fund (K 104724 and NN 117637). J. E. gratefully acknowledges the scholarship awarded by New National Excellence Program of the Ministry of Human Capacities (ÚNKP-16-3-III.).Notes and references
- S. Hjerten, J. L. Liao, K. Nakazato, Y. Wang, G. Zamaratskaia and H. X. Zhang, Chromatographia, 1997, 44, 227–234 CAS.
- R. X. Gao, X. Kong, X. Wang, X. W. He, L. X. Chen and Y. K. Zhang, J. Mater. Chem., 2011, 21, 17863–17871 RSC.
- T. Shiomi, M. Matsui, F. Mizukami and K. Sakaguchi, Biomaterials, 2005, 26, 5564–5571 CrossRef CAS PubMed.
- F. T. C. Moreira, S. Sharma, R. A. F. Dutra, J. P. C. Noronha, A. E. G. Cass and M. G. F. Sales, Biosens. Bioelectron., 2013, 45, 237–244 CrossRef CAS PubMed.
- J. Bognár, J. Szűcs, Z. Dorkó, V. Horváth and R. E. Gyurcsányi, Adv. Funct. Mater., 2013, 23, 4703–4709 CrossRef.
- A. Cutivet, C. Schembri, J. Kovensky and K. Haupt, J. Am. Chem. Soc., 2009, 131, 14699–14702 CrossRef CAS PubMed.
- A. Bossi, M. Andreoli, F. Bonini and S. Piletsky, Anal. Bioanal. Chem., 2007, 389, 447–454 CrossRef CAS PubMed.
- B. Zdyrko, O. Hoy and I. Luzinov, Biointerphases, 2009, 4, Fa17–Fa21 CrossRef CAS PubMed.
- E. Komarova, M. Aldissi and A. Bogomolova, Analyst, 2015, 140, 1099–1106 RSC.
- A. P. M. Tavares, F. T. C. Moreira and M. G. F. Sales, RSC Adv., 2013, 3, 26210–26219 RSC.
- M. S. R. Khan, F. T. C. Moreira, J. Riu and M. G. F. Sales, Sens. Actuators, B, 2016, 233, 697–704 CrossRef CAS.
- F. T. C. Moreira, S. Sharma, R. A. F. Dutra, J. P. C. Noronha, A. E. G. Cass and M. G. F. Sales, Sens. Actuators, B, 2014, 196, 123–132 CrossRef CAS.
- A. R. Chaves and M. E. C. Queiroz, J. Chromatogr. A, 2013, 1318, 43–48 CrossRef CAS.
- K. Lee, R. R. Itharaju and D. A. Puleo, Acta Biomater., 2007, 3, 515–522 CrossRef CAS PubMed.
- S. A. Yu, A. Q. Luo, D. Biswal, J. Z. Hilt and D. A. Puleo, Talanta, 2010, 83, 156–161 CrossRef CAS PubMed.
- J. T. Huang, J. Zhang, J. Q. Zhang and S. H. Zheng, J. Appl. Polym. Sci., 2005, 95, 358–361 CrossRef CAS.
- D. L. Venton and E. Gudipati, Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol., 1995, 1250, 126–136 CrossRef.
- H. Taguchi, H. Sunayama, E. Takano, Y. Kitayama and T. Takeuchi, Analyst, 2015, 140, 1448–1452 RSC.
- K. El Kirat, M. Bartkowski and K. Haupt, Biosens. Bioelectron., 2009, 24, 2618–2624 CrossRef CAS PubMed.
- W. X. Su, J. Rick and T. C. Chou, Microchem. J., 2009, 92, 123–128 CrossRef CAS.
- D. M. Hawkins, D. Stevenson and S. M. Reddy, Anal. Chim. Acta, 2005, 542, 61–65 CrossRef CAS.
- M. Zayats, M. Kanwar, M. Ostermeier and P. C. Searson, Macromolecules, 2011, 44, 3966–3972 CrossRef CAS.
- A. Bossi, S. A. Piletsky, E. V. Piletska, P. G. Righetti and A. P. F. Turner, Anal. Chem., 2001, 73, 5281–5286 CrossRef CAS PubMed.
- M. Fountoulakis, J. Chem. Technol. Biotechnol., 1995, 62, 81–90 CrossRef CAS.
- B. Heras, S. R. Shouldice, M. Totsika, M. J. Scanlon, M. A. Schembri and J. L. Martin, Nat. Rev. Microbiol., 2009, 7, 215–225 CrossRef CAS PubMed.
- J. D. Radolf, L. A. Borenstein, J. Y. Kim, T. E. Fehniger and M. A. Lovett, J. Bacteriol., 1987, 169, 1365–1371 CrossRef CAS PubMed.
- T. S. Raju and B. J. Scallon, Biochem. Biophys. Res. Commun., 2006, 341, 797–803 CrossRef CAS PubMed.
- C. N. Yao, L. Huiying, S. Pengjun, H. Huoqing, W. Yaru, Y. Peilong and Y. Bin, Appl. Environ. Microbiol., 2016, 82, 1004–1014 CrossRef PubMed.
- W. Stöber, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef.
- G. H. Bogush, M. A. Tracy and C. F. Zukoski, J. Non-Cryst. Solids, 1988, 104, 95–106 CrossRef CAS.
- PeptideCutter, http://web.expasy.org/peptide_cutter/.
- K. G. Welinder, Eur. J. Biochem., 1979, 96, 483–502 CrossRef CAS PubMed.
- Y. Q. Yu, M. Gilar, P. J. Lee, E. S. P. Bouvier and J. C. Gebler, Anal. Chem., 2003, 75, 6023–6028 CrossRef CAS PubMed.
- Y. Seo, S. Sato, K. Kuroki and T. Kishida, Forensic Sci. Int., 2013, 232, 154–159 CrossRef CAS PubMed.
- M. Bergallo, C. Costa, G. Gribaudo, S. Tarallo, S. Baro, A. N. Ponzi and R. Cavallo, New Microbiol., 2006, 29, 111–119 CAS.
| This journal is © The Royal Society of Chemistry 2017 |