Raman spectroscopy and multivariate analysis for the non invasive diagnosis of clinically inconclusive vulval lichen sclerosus
Jonathan
Frost
 *ab,
Linmarie
Ludeman
c,
Kathryn
Hillaby
c,
Robert
Gornall
c,
Gavin
Lloyd
b,
Catherine
Kendall
b,
Angela C.
Shore
d and
Nick
Stone
a
*ab,
Linmarie
Ludeman
c,
Kathryn
Hillaby
c,
Robert
Gornall
c,
Gavin
Lloyd
b,
Catherine
Kendall
b,
Angela C.
Shore
d and
Nick
Stone
a
aBiomedical Physics, School of Physics and Astronomy, University of Exeter, Exeter, EX4 4QL, UK. E-mail: j.frost@nhs.net; Fax: +44 (0)3004225485; Tel: +44 (0)3004225473
bBiophotonics Research Unit, Gloucestershire Hospitals NHS Foundation Trust, Great Western Road, Gloucestershire, GL1 3NN, UK
cCheltenham General Hospital, Gloucestershire Hospitals NHS Foundation Trust, Sandford Road, Gloucestershire, GL53 7AN, UK
dMedical School and NIHR Exeter Clinical Research Facility, University of Exeter, Exeter, EX4 4QL, UK
First published on 3rd November 2016
Abstract
Vulval lichen sclerosus (LS) is a common inflammatory condition associated with an increased risk of developing vulval carcinoma. Diagnosis is usually clinical although biopsy is necessary if the diagnosis is uncertain or if there is a failure to respond to adequate initial treatment. Raman spectroscopy has the potential to be applied in vivo for near real time objective non-invasive optical diagnosis, avoiding the need for invasive tissue biopsies. The aim of this study was to evaluate the diagnostic performance of Raman spectroscopy for differentiating LS from other vulval conditions in fresh vulval biopsies. Biopsies were analysed from 27 women with suspected LS in whom the attending gynaecologist could not establish the diagnosis on clinical presentation alone. Spectral variance was explored using principal component analysis and in conjunction with the histological diagnoses was used to develop and test a multivariate linear discriminant classification model. This model was validated with leave one sample out cross validation and the diagnostic performance of the technique assessed in comparison with the pathology gold standard. After cross validation the technique was able to correctly differentiate LS from other inflammatory vulval conditions with a sensitivity of 91% and specificity of 80%. This study demonstrates Raman spectroscopy has potential as a technique for in vivo non-invasive diagnosis of vulval skin conditions. Applied in the clinical setting this technique may reduce the need for invasive tissue biopsy. Further in vivo study is needed to assess the ability of Raman spectroscopy to diagnose other vulval conditions before clinical application.
Introduction
Lichen sclerosus (LS) is an inflammatory condition that can affect any skin site but is most commonly found in the vulva.1 The true prevalence of LS is unknown, however community studies in the elderly have demonstrated a prevalence as high as 3%.2The aetiology of LS is not well described although there is some evidence to suggest that the LS has an autoimmune aetiology.3 It has been observed that autoimmune conditions are more prevalent in women with LS compared unaffected women.4 In addition, antibodies to extracellular proteins have been demonstrated in women with this condition.5 Histological changes can be subtle but typically shows a thinned epidermis, hyalinisation of the superficial collagen matrix with a deeper lymphohistiocytic inflammatory infiltrate.
Characteristic symptoms of LS include pruritis, irritation and soreness although the condition can be asymptomatic. The condition typically appears pale and atrophic although blistering, erosions, purpura and hyperkeratosis can be present. Vulval scarring leads to destruction of normal anatomical features and can result in fusion of the labia, stenosis of the entrance to the vaginal as well as burying of the clitoris.1 This scarring leads to significant physical, psychological and psychosexual morbidity in those affected.6
LS is complicated by development of squamous cell carcinoma (SCC) in approximately 4% of women.1,7 This compares to a background risk of vulval cancer in the UK of 0.3%.8
Vulval LS may occur at any age but typically either appears in prepubertal girls or post menopausal women.9 Diagnosis can be clinical although invasive tissue biopsy and histopathological evaluation is necessary if the diagnosis is uncertain or if there is a failure to respond to adequate treatment.10 Histopathological examination is considered to be the gold standard diagnostic procedure, however the process is time consuming, expensive and subject to sampling errors as the number of areas analysed is limited by the morbidity of the biopsy procedure.
The focus of treatment is on symptom resolution and halting the progressive scarring of the disease. The mainstay of this treatment is ultrapotent topical corticosteroids applied to the affected area. Most patients will respond to topical corticosteroids with clinical improvement and histological regression. Remission of symptoms occurs in ninety five percent of women and maintenance treatment has the potential to reduce risk of progression to vulval cancer without significant adverse effects.10–12 The exact mechanism leading to the malignant transformation in LS has not been well described. Observation of the development of LS associated carcinoma appears to suggest carcinogenesis is dependent on stage, duration and activity of the disease.13,14 One hypothesis suggests that the immune dysregulation present in LS creates a permissive environment for the development of vulval SCC.13,14 For this reason it has been suggested that controlling the immune response and resolving the histological changes with ultrapotent topical corticosteroids will reduce the risk of malignant transformation, although evidence from clinical trials is currently lacking. A recent survey of UK clinicians involved in the care of women with vulval cancer found that a majority of respondents prescribed topical steroids indefinitely in women who have been treated for SCC with concurrent LS to prevent recurrence of SCC.15 Currently the success of treatment is primarily judged through symptomatic response and not histological or clinical regression of the disease. As the condition can be asymptomatic this approach may result in treatment regimens that do not suppress the underlying pathological inflammatory process, permitting progressive scarring and a cellular environment conducive to carcinogenesis.
After initial treatment patients require long term follow up either with regular medical review or self monitoring by the patient due the increased risk of progression to squamous cell carcinoma.16 If vulval cancer is suspected then biopsy is required.
There are currently no non-invasive alternatives to invasive biopsy for diagnosis of LS or monitoring response to treatment. A reliable non-invasive diagnostic technique would reduce the need for invasive biopsy and allow a greater area of the vulva to be evaluated. In addition, a non-invasive technique for objectively monitoring biochemical response to treatment may allow clinicians to tailor treatment to a reduction in the pathological inflammatory process, which may in turn reduce the risk of progression to vulval SCC.
Optical techniques provide a practical approach for non-invasive, real time diagnosis and monitoring of vulval skin lesions. Raman spectroscopy is an optical technique that can be used to evaluate the biomolecular composition of tissue by assessing the vibrational energy levels of the constituent molecules.17 Within the Raman spectrum, individual peaks correspond to specific molecular bonds or groups of bonds. As Raman spectroscopy is able to detect the biochemical changes associated with different pathological processes it can be applied as a in vivo non-invasive diagnostic technique.18–20 Multiple studies have demonstrated the ability of Raman spectroscopy to differentiate between a number of dermatological conditions, including psoriasis, basal cell carcinomas, actinic keratosis, and benign & malignant pigmented lesions.21–25 The affect of changes in collagen structure and inflammation within skin on the Raman spectra has also been established.26,27 These changes are key in the pathogenesis of LS and suggest Raman spectroscopy has the potential to differentiate LS from other skin disorders. To our knowledge there are no studies exploring the use of Raman spectroscopy for the diagnosis or monitoring of LS.
In this study we explore the role of Raman spectroscopy in the assessment of women suspected to have a diagnosis of LS in whom a clinical diagnosis could not be established with confidence. The spectral characteristics of LS are explored and the diagnostic performance assessed. In addition to analysis of the study group it would be desirable to investigate LS in patients in whom clinical diagnosis is possible to characterise the full range of the disease. In this preliminary ex vivo study this was not possible as taking invasive biopsies for research purposes alone was reported to be unacceptable to women during initial consultation with patient representatives. This also means that obtaining tissue for ex vivo analysis of a normal control group was unacceptable to women.
Experimental
Sample collection and preparation
All vulval tissue samples were obtained under a protocol approved by the NHS Health Research Authority, National Research Ethics Service Committee South West-Exeter (ref: 14/SW/1077). Women referred to specialist gynaecology services at Gloucestershire Hospitals NHS Foundation Trust for assessment of vulval complaints were considered for inclusion in the study. Clinicians providing specialist gynaecology care identified women in whom LS was the main differential diagnosis but who required invasive biopsy as part of their routine care due to diagnostic uncertainty. The women identified gave written consent for recruitment to the study. Vulval biopsies were collected using a 4 mm Keye's biopsy punch with physiological (0.9%) saline skin preparation and 2% lidocaine subdermal infiltration for anesthesia. This procedure results in a circular disc of skin 3–4 mm in diameter, comprising the full thickness of the epidermis with underlying dermal tissue. The location and number of biopsies collected was directed by clinical need rather than the requirements of the research. Biopsies were transported in physiological (0.9%) saline to the on-site spectroscopy laboratory for immediate analysis. After spectral analysis samples were marked with dye to allow orientation and fixed in formalin for histopathological analysis.Spectral analysis
Spectra were measured using a modified Renishaw system 1000 dispersion Raman micro-spectrometer optimised for tissue analysis in the near infrared range. The spectrometer excitation beam was an 830 nm diode laser with a power output of 330 mW resulting in power of 35 mW at the sample. The spectrometer was used in conjunction with a Leica confocal light microscope with a motorised stage using Leica x50 N Plan long working distance objective selected for minimal fluorescence and Raman signature. The laser spot had dimensions 15 × 17 μm. The spectrometer and stage were controlled by via a computer interface running Renishaw WIRE software (version 2.0, service pack 9). The entry slit to the spectrometer was set to 50 μm throughout. System calibration was performed using four standards: silicon wafer (A-9800-CACC, Renishaw Inc., Gloucestershire, UK), neon argon lamp (A-9800-CACC, Renishaw Inc., Gloucestershire, UK), cyclohexane (154741-1L, Sigma Aldrich, UK) and green glass. The green glass produced a broad fluorescent response and was used to correct for variation in instrument response when compared with the spectrum of a tungsten filament lamp calibrated at the National Physics Laboratory (Teddington, UK).The fresh unprocessed biopsies were subjected to near infrared Raman spectroscopy prior to routine histopathological assessment. To simulate in vivo application of the technique, spectra were collected from the epidermal surface of the vulval skin biopsies. Samples were mounted in a custom-built stainless steel tissue mount with the epidermis pressed against a 250 μm thick Raman grade calcium fluoride window. The mount was positioned on the microscope stage so that spectra could be acquired from the sample through the cover slip. Spectra were acquired in a line across the central 2 mm of the sample at intervals of 50 μm with the laser focused on the surface of the sample and an acquisition time of 20 seconds. In order to assess the optimal focal point for acquiring spectra in this type of tissue this process was repeated after the microscope stage was advanced towards the objective. The stage was advanced in steps of 15 μm and spectra measured at each position until the sample had been advanced 150 μm towards the objective. On average of 440 spectra were measured from each sample.
Histopathology
After spectral measurement the tissue sample was fixed in formalin and subjected to routine processing and staining in the pathology department. All samples underwent histopathological assessment by a specialist gynaecological pathologist blinded to the results of the spectroscopic analysis. Samples were grouped according to presence (group 1) or absence (group 2) of features consistent with a diagnosis of LS (Fig. 1).Data analysis
The data analysis was carried out using Matlab (2015a) Mathworks USA. Spectral data points outside of the 400 to 1800 cm−1 biomolecular fingerprint range were excluded from the analysis and all spectra underwent an instrument response correction using the spectrum measured from the green glass.28 The spectra were then vector normalized and autofluorescence removed using a second order polynomial fitting and subtraction.29 Cosmic rays were identified by isolating spectral points where the difference between the spectra and the median spectra for that sample at the given depth deviated from the median absolute difference by greater than 4 standard deviations. Where cosmic rays were identified the affected region of the spectrum was removed and substituted by a reconstruction using principal component loadings from analysis of spectra free from cosmic rays. The resultant spectra were then analysed with principal component analysis (PCA) to reduce the dimensionality of the data in preparation for linear discriminant analysis (LDA).The classification ability of Raman spectroscopy to detect whether a tissue sample was from a patient with LS (group 1) or another pathology (group 2) was evaluated using leave one sample out cross validation to reduce the probability of a type 1 error (false positive). A stepwise selection of principal components from the initial 24 produced by the model was used to reduce the significance of noise in the classification model and reduce the risk of a type 2 error (false negative).
In the stepwise cross validation cycle the data from a whole tissue sample (test set) was removed from the complete data set. After this a randomly selected 25% of the remaining full spectra were then removed from the data set as a stepwise sub test set. PCA fed LDA was then performed on the remaining data. The resultant model was then tested on the stepwise sub test set using each principal component (PC) in turn to identify the PC which resulted in the highest proportion of correct predictions. This PC then remained in the PCA–LDA model and the remaining PCs were tested alongside this PC in the same manner to identify the next best performing PC. This process was repeated adding the next best performing PC to the model each time. Only those PCs that improved the performance of the PCA–LDA model were then selected for classification of the whole sample test set.
The classification performance of the PCA–LDA model was assessed on its ability to correctly classify the histological diagnosis of the whole sample test set. This leave one out stepwise cross validation loop was then repeated evaluating each sample in turn.
The classification performance of individual spectra was assessed using the receiver operator characteristic to simulate measurements that could be acquired in a clinical setting. The probability of obtaining a false positive result was evaluated using the method described by Obuchowski et al.30,31 This process was repeated for the measurements taken at different focal depths to select the optimum focal position for correctly classifying the pathology within the tissue.
Results & discussion
Samples from 27 women with suspected LS were subject to spectroscopic and histopathological analysis. Of these, 15 women had a histological diagnosis of LS. Of the remaining 12 women, the majority had findings of nonspecific inflammation only, some had no specific histological abnormality and there was one case of mild dysplasia (Table 1).| Histological diagnoses | N | % |
|---|---|---|
| Lichen sclerosus | 15 | 56 |
| Other diagnoses | 12 | 44 |
| Non specific inflammation | (8) | |
| Mild dysplasia | (1) | |
| No specific histological abnormality | (3) |
Spectra were measured from all samples between 30 minutes and 7 hours of the samples being collected. A mean of 440 spectra were collected from each sample. A two group analysis of the data was performed to differentiate those samples from women with LS from those with other diagnoses as this was the most clinically relevant comparison possible using the available data.
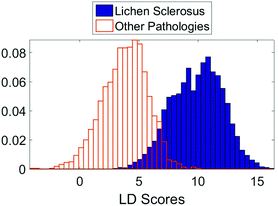
The initial PCA–LDA analysis demonstrated that the technique was able to correctly classify the majority of samples based on the spectra acquired using the linear discriminant scores (Fig. 2). This result was replicated in the leave one out stepwise cross validation. The bimodal distribution of linear discriminant scores in the lichen sclerosus group (Fig. 2) suggests there may be separate biomolecular subtypes of the disease although no correlation between the scores and histological features could be identified.
Analysis of the spectra from different focal points within the samples revealed the diagnostic performance was optimal when then focal point was advanced 15 microns into the samples. Only data from this depth (40 spectra from each sample) were used in the final analysis.
Defining the biochemical constituent peaks responsible for discrimination within the LDA model is challenging as Raman spectroscopy does not allow simple biochemical quantification of target tissues. This is due to the compound effect on the Raman spectra from the numerous molecular constituents of the tissue.
The dominant histological feature of LS is hyalinisation of the dermis typically seen alongside epidermal atrophy and subdermal inflammatory cell infiltrate. The dermis primarily consists of collagen fibres with smaller quantities of elastic fibres and other extracellular proteins. In human skin type 1 collagen accounts for 80% of all dermal collagen. In LS significant biochemical alterations occur around the zone of hyalinisation with changes in the organization and expression of type I, III collagen fibers, a relative increase in the expression of type V collagen and a decrease in the expression of extracellular matrix protein 1 and elastin. These changes may form the molecular basis for the differentiation of the two groups in this study.32
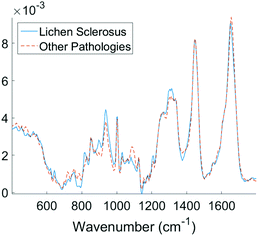
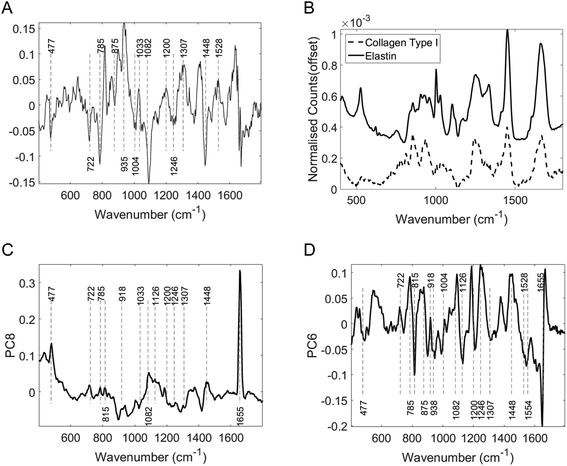
Examining the two most significant principal component loadings and the composite of all principal component loadings weighted by contribution to the linear discriminant classification model (Fig. 4) spectral differences between the two groups (Fig. 3) can be seen to occur in the regions associated with collagen, elastin and other structural proteins (Table 2). Changes are also seen in the lipid, fatty acid, nucleic acid and carbohydrate regions of the spectra (Table 2). These changes may well be associated with epidermal atrophy, vacuolar degeneration and the lichenoid lymphocytic inflammatory infiltrate seen in LS.
| Wavenumber (cm−1) | Suggested biomolecular constituent allocation |
|---|---|
| Wavenumber assignments from Talari et al. and Nguyen et al.17,33 | |
| Type 1 collagen | |
| 815 | C–O–C stretching |
| 722 | C–S stretching |
| 856 and 920 | Proline ring |
| 875 | Hydroxyproline ring |
| 938 | C–C stretching |
| 1004 & 1033 | Phenyalanine |
| 1043 | Proline |
| 1082 | Carbohydrate residues of collagen |
| 1668 | Amide I |
| 1246 | Proline rich region |
| 1271 | Proline poor region |
| 1448 | CH deformation |
| Nucleic acids | |
| 722 | DNA |
| 785 | DNA/RNA |
| 1325–1330 | Nucleic acids |
| Carbohydrates | |
| 477 | Polysaccharides |
| Other protein and amino acids | |
| 918 | Proline, hydroxyproline |
| 935 | C–C stretching mode of proline and valine and protein backbone/glycogen |
| 1000 | Phenylalanine |
| 1053 | C–O stretching, C–N stretching (protein) |
| 1126 | C–N stretching vibration (protein vibration) |
| 1200–1300 | Amide III (proteins) |
| 1307 | CH3/CH2 twisting, wagging, and/or bending mode of collagens and lipids |
| 1528 | Carotenoid |
| 1554 | Amide II |
Analysis of diagnostic performance after cross validation demonstrated Raman spectroscopy combined with multivariate analysis was able to differentiate vulval samples from women with LS from those with other diagnoses with a sensitivity of 91% and specificity of 80%. The area under the receiver operator curve was 0.95 with a false positive rate of <0.05 (Fig. 5).
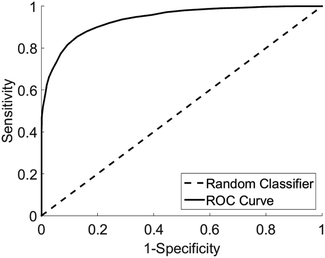 | ||
| Fig. 5 Receiver operator curve for the detection of LS from other pathologies, area under curve 0.95, type 1 error rate <0.05. | ||
Conclusions
This proof of concept diagnostic study demonstrates Raman spectroscopy combined with multivariate analysis has real potential as a useful tool in the diagnosis and monitoring of women with lichen sclerosus, a common vulval skin condition. In this study of patients who required invasive tissue biopsy for diagnosis Raman spectroscopy was able to correctly diagnose LS with clinically applicable performance.Analysing spectra on a per patient basis and selecting a diagnostic threshold that optimizes for specificity, a low false positive rate can be achieved with a clinically relevant true negative rate. This approach was able to correctly identify all 12 patients that did not have LS (test negative) with a negative predictive value of 0.8.
In clinical practice only those with an uncertain diagnosis require invasive biopsy. These results suggest that by using Raman spectroscopy and avoiding biopsy in those who have a positive optical diagnosis of LS we could expect to reduce the number of invasive biopsies required. Using this approach in the study group only the 66% of patients that tested negative for LS would require a biopsy reducing the number of invasive biopsies required by 44%. This has the potential to reduce the morbidity for the women; reduce the time taken to diagnose the condition and reduce the significant direct and indirect costs of invasive biopsy. In addition, this technique would allow non-invasive assessment of multiple regions of the vulva and would not be limited by the morbidity associated with invasive biopsy.
After further study Raman spectroscopy may provide a valuable technique for the objective assessment of response to treatment of women with asymptomatic vulval LS. The technique could be used to direct treatment towards biochemical and histological rather than symptomatic control of disease. This in turn may prevent the development of a cellular environment conducive to malignant transformation.
Further in vivo research is required to validate this technique using probe based systems and to assess performance across a wider range of vulval pathologies. The design of probes for in vivo trials should consider that laser focus beneath the surface of the skin may yield improved diagnostic performance, compared to probes that focus at the surface. Given the association between LS and vulval cancer it is important that future studies include patients with vulval cancer to ensure these women can be correctly identified. This could be achieved by analysis of tissue from women undergoing biopsy or excisional treatment of vulval pre-cancer or cancer.
Conflicts of interest/sources of support
All authors report a grant received from British Society for the Study of Vulval Disease which part funded the conduct of the study.Acknowledgements
We acknowledge the British Society for the Study of Vulval Disease and the Gloucestershire Hospitals NHS Foundation Trust Research and Innovation Forum for financial support. We thank Jo Motte and the team at the Gloucestershire Hospitals NHS Foundation Trust histopathology department for the preparation of the tissue sections. The study was supported by the NIHR Exeter Clinical Research Facility. The interpretations of data in the paper are those of the authors and not of NIHR or the Department of Health.References
- J. Powell and F. Wojnarowska, Lancet, 1999, 353, 1777–1783 CrossRef CAS.
- A. Leibovitz, V. Kaplun, N. Saposhnicov and B. Habot, Arch. Gerontol. Geriatr., 2000, 31, 1–4 CrossRef PubMed.
- R. H. Meyrick Thomas, C. M. Ridley, D. H. Mcgibbon and M. M. Black, Br. J. Dermatol., 1988, 118, 41–46 CrossRef CAS PubMed.
- C. I. Harrington and I. R. Dunsmore, Br. J. Dermatol., 1981, 104, 563–566 CrossRef CAS PubMed.
- N. Oyama, I. Chan, S. Neill and T. Hamada, Lancet, 2003, 362, 118–123 CrossRef CAS.
- H. K. Haefner, N. Z. Aldrich, V. K. Dalton, H. M. Gagné, S. B. Marcus, D. A. Patel and M. B. Berger, J. Womens Health, 2014, 23, 765–770 CrossRef PubMed.
- J. a. Carlson, R. Ambros, J. Malfetano, J. Ross, R. Grabowski, P. Lamb, H. Figge and M. C. Mihm, Hum. Pathol., 1998, 29, 932–948 CrossRef CAS PubMed.
- Cancer Research UK, 2016.
- J. J. Meffert, B. M. Davis and R. E. Grimwood, J. Am. Acad. Dermatol., 1995, 32, 393–416 CrossRef CAS PubMed.
- S. Neill and F. Lewis, Br. J. Dermatol., 2010, 672–682 CrossRef CAS PubMed.
- C.-C. Chi, G. Kirtschig, M. Baldo, F. Lewis, S.-H. Wang and F. Wojnarowska, J. Am. Acad. Dermatol., 2012, 67, 305–312 CrossRef PubMed.
- S. K. Fistarol and P. H. Itin, Am. J. Clin. Dermatol., 2013, 14, 27–47 CrossRef PubMed.
- B. Brodrick, Z. R. Belkin and A. T. Goldstein, Clin. Dermatol., 2013, 31, 780–786 CrossRef PubMed.
- S. Regauer, Eur. J. Cell Biol., 2005, 84, 273–277 CrossRef CAS PubMed.
- T. Sanna, R. Pounds, J. Yap and D. Luesley, in RCOG World Congress, 2016.
- T. Crowley, RCOG, 2014, 1–35.
- A. C. S. Talari, Z. Movasaghi, S. Rehman and I. Rehman, Appl. Spectrosc. Rev., 2015, 50, 46–111 CrossRef CAS.
- O. J. Old, L. M. Fullwood, R. Scott, G. R. Lloyd, L. M. Almond, N. A. Shepherd, N. Stone, H. Barr and C. Kendall, Anal. Methods, 2014, 6, 3901 RSC.
- J. J. Wood, C. Kendall, J. Hutchings, G. R. Lloyd, N. Stone, N. Shepherd, J. Day and T. A. Cook, Color. Dis., 2014, 16, 732–738 CrossRef CAS PubMed.
- H. Lui, J. Zhao, D. McLean and H. Zeng, Cancer Res., 2012, 72, 2491–2500 CrossRef CAS PubMed.
- T. R. Hata, T. a. Scholz, I. V. Ermakov, R. W. McClane, F. Khachik, W. Gellermann and L. K. Pershing, J. Invest. Dermatol., 2000, 115, 441–448 CrossRef CAS PubMed.
- M. Gniadecka, O. Faurskov Nielsen, D. H. Christensen and H. C. Wulf, J. Invest. Dermatol., 1998, 110, 393–398 CrossRef CAS PubMed.
- M. Gniadecka, H. C. Wulf and N. N. Mortensen, J. Raman Spectrosc., 1997, 28, 125–129 CrossRef CAS.
- S. Sigurdsson, P. A. Philipsen, L. K. Hansen, J. Larsen, M. Gniadecka and H. C. Wulf, IEEE Trans. Biomed. Eng., 2004, 51, 1784–1793 CrossRef PubMed.
- A. Nijssen, T. C. Bakker TC, F. Heule, P. J. Caspers, D. P. Hayes, M. H. Neumann and G. J. Puppels, J. Invest. Dermatol., 2002, 119, 64–69 CrossRef CAS PubMed.
- L. Knudsen, C. K. Johansson, P. A. Philipsen, M. Gniadecka and H. C. Wulf, J. Raman Spectrosc., 2002, 33, 574–579 CrossRef CAS.
- C. a. Lieber, S. K. Majumder, D. L. Ellis, D. D. Billheimer and A. Mahadevan-Jansen, Lasers Surg. Med., 2008, 40, 461–467 CrossRef PubMed.
- M. C. M. Grimbergen, C. F. P. Van Swol, C. Kendall, R. M. Verdaasdonk, N. Stone and J. L. H. R. Bosch, Appl. Spectrosc., 2010, 64, 8–14 CrossRef CAS PubMed.
- C. a. Lieber and A. Mahadevan-Jansen, Appl. Spectrosc., 2003, 57, 1363–1367 CrossRef CAS PubMed.
- N. A. Obuchowski, M. L. Lieber and F. H. Wians, Clin. Chem., 2004, 50, 1118–1125 CAS.
- N. A. Obuchowski and D. K. McClish, Stat. Med., 1997, 16, 1529–1542 CrossRef CAS PubMed.
- C. Godoy, W. Teodoro, A. Velosa, A. Garippo, E. Eher, E. Parra, M. Sotto and V. Capelozzi, Clinics, 2015, 70, 356–362 CrossRef PubMed.
- T. T. Nguyen, C. Gobinet, J. Feru, S. Brassart-Pasco, M. Manfait and O. Piot, Adv. Biomed. Spectrosc., 2013, 7, 105–110 CAS.
| This journal is © The Royal Society of Chemistry 2017 |