Open Access Article
Open Access ArticlePreparation of high oil-absorptive uniform gel with controllable oil-absorbency by radiation
Minghua Zhanga,
Minmin Fan a,
Xuewu Geb,
Ke Wang*a and
Xi Zhang*a
a,
Xuewu Geb,
Ke Wang*a and
Xi Zhang*a
aState Key Laboratory of Polymer Materials Engineering, Polymer Research Institute of Sichuan University, Sichuan University, Chengdu 610065, China. E-mail: kewang33@vip.sina.com; zhangxi@scu.edu.cn
bDepartment of Polymer Science and Engineering, University of Science and Technology of China, Hefei, Anhui 230026, China
First published on 20th June 2017
Abstract
A series of 4-t-butyl styrene-g-ethylene propylene diene monomer (EPDM) (t-BS-g-EPDM) gels was prepared by direct gamma ray irradiation with varying contents of t-BS in EPDM. Characterization of irradiated t-BS-g-EPDM gels was performed by Fourier transform infrared (FTIR) spectroscopy, tensile tests, dynamic mechanical analysis (DMA), thermogravimetric analysis (TGA), scanning electron microscopy (SEM), and oil absorbency tests. It was observed that the increment in irradiation dose up to 90 kGy would increase the percentage of gel content due to the increase in crosslink density. Tensile properties of t-BS-g-EPDM gels increased with the introduction of irradiation up to 150 kGy except for elongation at break. The section picture of t-BS-g-EPDM gels by SEM showed a homogenous and smooth surface, indicating no phase separation upon irradiation. The t-BS-g-EPDM gels exhibited high swelling ability in organic solvents, and the maximum values of oil absorbency to chloroform and diesel oil were 42.3 g g−1 and 23.6 g g−1, respectively. These results indicated that the properties of t-BS-g-EPDM gels have been improved by irradiation with respect to an irradiated EPDM sample.
1. Introduction
In the research of the past decades, oil absorptive polymers with three dimensional cross-linked networks have attracted a great deal of attention because of the oil pollution of marine environments becoming more and more serious with the development of the petroleum industry and marine oil transportation.1 And, the development of EPDM used for oil absorptive gels2 is increasing due to its non-polar nature3 and excellent resistance to heat, light, oxygen, and ozone.4 Since the double chemical bonds in EPDM can be easily broken to produce monomer radicals and then cause crosslinking reaction upon irradiation, EPDM is also frequently used as a crosslinkable polymer under ionizing radiation.A series of EPDM-based oil absorptive polymers has been synthesized successfully, achieving high oil absorptive gels which can be used for commercial purposes.5–8 Among them, bead-shape P(t-BS-EPDM) oil gels prepared by suspension polymerization, are of good oil absorption, which are also convenient for processing, shipping and storage. However, it is impossible to control the shape of the product according to their practical applications. Moreover, the conventional organic solvents used in suspension and solution polymerization have detrimental effects on the environment and human health. Therefore, the need to develop a green and low cost-consuming technology for the production of oil-absorptive gels is paramount; the leading candidate is radiation.
Radiation is the unique source of energy which can initiate chemical reactions at any temperature, including ambient, under any pressure, in any phase (gas, liquid or solid), without use of catalysts.9 Compared to other crosslinking methods, crosslinking by radiation is a simple and an eco-friendly process which has already been commercialized.9–11 Radiation processing of nanocomposites using high-energy radiation is expected to provide uniformly cross-linked networks in comparison to conventionally vulcanized networks.12,13 The low cost of operation, additive-free technique, and room-temperature operations are among the additional advantages of radiation cross-linking over the existing other cross-linking techniques.14
Our recent research15 provided a new reactive processing method to prepare 4-t-butyl styrene–ethylene propylene diene monomer–divinyl benzene (t-BS–EPDM–DVB) oil absorptive gel without using organic solvents. However, the maximum chloroform absorbency of the resulting oil absorptive gels was 15.9 g g−1, which should be further enhanced in the future. In the present study, radiation technique has been used to prepare crosslinked oil absorptive gels based on t-BS-g-EPDM polymer, wherein the effect of t-BS on the radiation vulcanization was investigated. Further, the present paper reports a detailed investigation on the effect of t-BS addition and radiation-induced cross-linking on the oil absorbency of various organic solvents of t-BS-g-EPDM gels. The final purpose of this work is to develop a convenience and efficient method to produce oil-absorptive gels with desirable shape, to reduce manufacturing costs of oil-absorptive gels, and to effectively widen the application field of oil absorptive gels by accurate control of oil-absorbency of products.
2. Materials and methods
2.1. Materials
Ethylene–propylene–diene copolymer (EPDM 7001) containing 73 wt% ethylene and 5 wt% ethylidenenorbornene was supplied by DuPont Dow Elastomer (USA). 4-t-Butyl styrene (t-BS; Aldrich Chem, USA) was purified prior to use. All other chemicals and reagents were of analytical grade and were used without further purification.2.2. Preparation of samples for irradiation
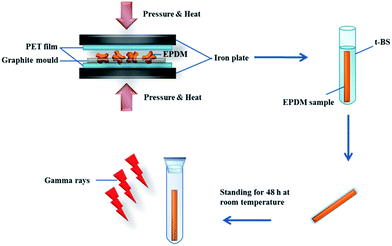
Firstly, EPDM rubber was heated to 180 °C and pressed under 20 MPa for 10 min, and then cold to room temperature under 10 MPa kept for 3 min into sheets of size 12 × 12 cm2 with thickness in the range of 1–4 mm. Rectangle-shaped specimens (width of 4 mm and length of 15 mm) were cut from the sheets. And then, the resulting EPDM samples were soaked in t-BS for different time to prepare a series of t-BS/EPDM blends containing different amount of t-BS. Finally, the resulting samples were sealed in glass tube, and allowed to stand for 72 h prior to γ-irradiation under airtight conditions.2.3. Procedure of gamma ray irradiation
Irradiation of the prepared blends was carried out using 60Co gamma irradiator (model JS-7900, IR-148) under airtight conditions at a dose rate of 10 kGy h−1 with total irradiation doses varying from 10 to 250 kGy. The irradiated t-BS-g-EPDM (ESR) samples were recorded as ESR5, ESR10, ESR15, ESR20, ESR25, and the number suffixed to ESR represented the t-BS weight percentage used. The irradiated EPDM sample was also made by the same method as described above.2.4. Gel fraction
The gel fraction was measured by extracting the irradiated samples with tetrahydrofuran (THF) in a Soxhlet apparatus for 24 h. The remaining insoluble portion was dried in a vacuum oven at 60 °C last to a constant weight. Gel fraction was evaluated using the eqn (1):| Gel fraction (%) = W1/W0 × 100% | (1) |
2.5. Fourier transform infrared spectroscopy
Fourier transform infrared (FTIR) spectra of the samples before and after irradiation were recorded using a Nicolet 560 FTIR spectrometer (USA) at a 4 cm−1 resolution over the range of 4000–400 cm−1 with air as background.2.6. Dynamic mechanical analysis
Dynamic mechanical analysis (DMA) of samples was carried out on a TA Q800 DMA (TA Instruments, USA) instrument with heating rate of 3 °C min−1 from −70 to 100 °C under nitrogen atmosphere, and at frequency of 1 Hz. Specimens for the measurements were in size of 20 mm in length, 4 mm in width, and 1 mm in thickness. The glass transition temperature (Tg) was determined from the peak of the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ curve.
δ curve.
2.7. Tensile properties
Tensile properties of samples were measured by an Instron universal material testing machine (Instron 4502, Instron Corporation, MA, USA) using a crosshead speed of 100 mm min−1 at room temperature. Five specimens of each sample were tested under the same conditions and results were averaged for analysis.2.8. Thermogravimetric analysis (TGA)
The thermal behavior of samples was analyzed by applying a TGA Q50 (TA Instruments, USA) analyzer, at a heating rate of 10 °C min−1 from 30 to 600 °C under nitrogen atmosphere.2.9. Scanning electron microscopy
The sections of all samples were examined using a scanning electron microscopy (SEM) (Jeol JSM-5900LV, Japan) at an accelerating voltage of 20 kV. Cross sections of specimens were sputtered with a 10 nm thick layer of gold prior to SEM observations.2.10. Oil-absorption test
Oil-absorbencies of all samples were determined according to ASTMF726-81: 0.1 g of sample was placed in a stainless steel cage (4 × 4 × 2 cm3) and then immersed in different organic solvents (hexane, toluene, chloroform, diesel oil, and cyclohexane). The swollen samples were taken out at regular time intervals, tapped with filter paper gently, weighed on a balance and put it back into the original bath. This procedure of swelling and weighing was repeated until the weight of sample no longer increased. The equilibrium oil-absorbency of sample (Qeq) was calculated by the eqn (2):| Qeq = (We − W0)/W0 | (2) |
3. Results and discussion
The preparation of oil-absorptive gels by irradiation method is illustrated in Fig. 1. To obtain a high oil absorptive gel, rigid monomer t-BS is introduced to EPDM matrix; it proposed that the steric effect of butyl group of t-BS may provide the crosslinked polymer with a large cavity in which oil could fill. A uniform distribution of t-BS along the chain of EPDM matrix can be easily achieved because of the hydrophobic interaction of t-BS with EPDM matrix. Consequently, the grafting polymerization of t-BS onto EPDM can occur upon their exposure to high energy gamma-ray radiation to form a covalently crosslinked structure.3.1. Characterizations of the ESR gels
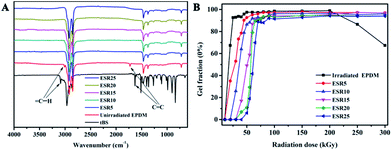
Fig. 2A shows the FTIR spectra of t-BS, unirradiated EPDM and ESR produced by 60 kGy radiation doses. The characteristic absorption bands of t-BS occur at 838 cm−1 corresponding to C–H bending vibration of the benzene ring, 902 cm−1 and 989 cm−1 corresponding to C–H bending vibration of –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, 1363 cm−1, 1402 cm−1 and 1463 cm−1 corresponding to C–H stretching vibration of –CH3, 1630 cm−1 corresponding to C
CH2, 1363 cm−1, 1402 cm−1 and 1463 cm−1 corresponding to C–H stretching vibration of –CH3, 1630 cm−1 corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of –CH
C stretching vibration of –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, and 3030 cm−1 and 3087 cm−1 corresponding to C–H stretching vibration of –CH
CH2, and 3030 cm−1 and 3087 cm−1 corresponding to C–H stretching vibration of –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, which are absent in the spectrum of unirradiated EPDM. After irradiation and purification by extraction, the ESR samples showed all characteristic absorption bands described above at the same wavenumber locations. However, the peak assigned to the vinyl bending and stretching vibration peak of pure t-BS at 902 cm−1, 989 cm−1, 1630 cm−1, 3030 cm−1 and 3087 cm−1 almost disappeared. And, a slight change in the intensity of the absorption peak due to the variation of t-BS content had no effect on the peak position. The results provided substantial evidence for the success of grafting t-BS onto EPDM chains through the direct irradiation method.
CH2, which are absent in the spectrum of unirradiated EPDM. After irradiation and purification by extraction, the ESR samples showed all characteristic absorption bands described above at the same wavenumber locations. However, the peak assigned to the vinyl bending and stretching vibration peak of pure t-BS at 902 cm−1, 989 cm−1, 1630 cm−1, 3030 cm−1 and 3087 cm−1 almost disappeared. And, a slight change in the intensity of the absorption peak due to the variation of t-BS content had no effect on the peak position. The results provided substantial evidence for the success of grafting t-BS onto EPDM chains through the direct irradiation method.
 | ||
| Fig. 2 (A) The FTIR spectra of t-BS, unirradiated EPDM and ESR produced by 60 kGy radiation dose. (B) Gel fraction of irradiated EPDM and ESR as a function of radiation dose. | ||
In order to characterize the irradiation reaction, the crosslinking degree of ESR was evaluated as a function of the radiation dose by gel content analysis. As shown in Fig. 2B, the gel content of the ESR gels with different t-BS content increased with irradiation dose up to 90 kGy and reached to a plateau at higher dose. This increase in gel content of the samples with irradiation dose can be attributed to the formation of radiation-induced crosslinked network among EPDM chains and with t-BS, indicating increases in crosslink density of the polymer. This phenomenon was also observed in other irradiation crosslinked polymer systems reported by other researchers.16 And, the occurrence of competing reactions of degradation and crosslinking in the polymer was the probable reason for the observed pattern at higher irradiation dose. For the irradiated EPDM, the gel fraction increased reaching its maximum values with increasing the irradiation dose up to about 200 kGy. After that, the values of gel fraction decreased sharply with increasing the irradiation dose. This may be due to predominant degradation taking place at the same time with crosslinking at higher irradiation doses.17,18 Moreover, the gel fraction decreased with increasing t-BS content at the same irradiation dose, which probably caused by the decreasing diene contents of graft or crosslinking site in EPDM as EPDM content decrease.
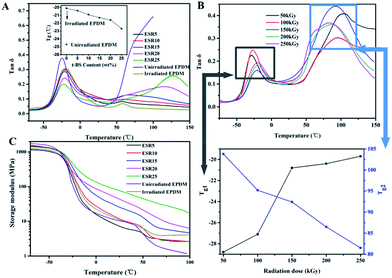
Generally, introduction of irradiation-induced crosslink into a polymer will proportionally increase the intermolecular network, resulting a rise in the glass transition temperature.11 For the irradiated EPDM sample, Tg peak shifted to higher temperature after irradiation at 90 kGy, which attributed to the increase in crosslink density (Fig. 3A). However, the Tg values for irradiated ESR gels (90 kGy) decreased with the increase of the t-BS content. These results can be attributed to the decrease in crosslink density of the ESR gel with increase in the t-BS content as observed in gel content result, indicating the formation of a loose copolymer network, which eventually facilitated the mobility of the polymer chains. Furthermore, with the increase of irradiation dose up to 250 kGy, the rise in Tg1 of EPDM and drop in Tg2 of PtBS homopolymer was noticed in ESR25 gel (Fig. 3B). This effect may be due to the occurrence of higher level of cross-linking in EPDM and t-BS with the increase of irradiation dose, which resulting the increase in compatibility between EPDM and t-BS phases.
The dynamic storage modulus was shown in Fig. 3C as a function of temperature for unirradiated EPDM, irradiated EPDM and ESR, which clearly indicated that storage modulus (E′) of all irradiated gels were higher than that of unirradiated EPDM. Storage modulus of ESR increased in accordance with t-BS content, due to the synergism effect of rigid phenyl structure and restrictions in the chain mobility. This means that the incorporation of t-BS into EPDM matrix remarkably enhances stiffness and load bearing capability of the material. Furthermore, it should be noted that the unirradiated EPDM is readily broken at high temperature (about 100 °C), in contrast with that irradiated EPDM and all ESR samples are more flexible and cannot be broken at 100 °C, which probably due to the formation of networks upon irradiation.
The morphology of ESR gels was observed using scanning electron microscope (SEM), in which the light regions correspond to hard phase (t-BS) and the dark regions correspond to soft phase (EPDM) (Fig. 4). This provides another proof of the dispersion of t-BS chains in the EPDM matrix. The SEM images showed the surface were homogeneous, smooth and exhibited no indication of phase separation after crosslinking. As seen in micrographs of ESR, the t-BS are distributed throughout the matrix with fewer or no clusters at low t-BS concentration (5 wt%, 10 wt%, and 15 wt%) stages, but at higher concentration stage some clusters emerges.
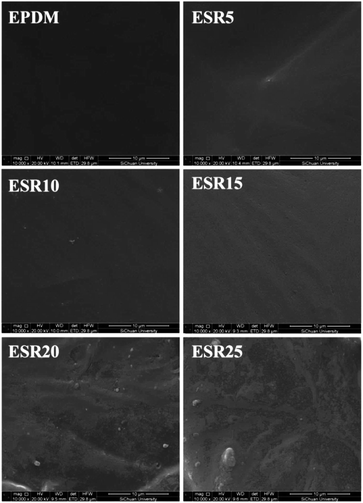 | ||
| Fig. 4 SEM images of the morphology of irradiated EPDM, ESR5, ESR10, ESR15, ESR20, and ESR25 (90 kGy). | ||
3.2. Mechanical properties of the ESR gels
Tensile strength is important for a material that is going to be stretched or under tension. Fig. 5A shows the tensile strength of the ESR increased with increasing of irradiation dose up to 150 kGy. This is due to the enhancement of the crosslink density,19,20 as indicated by the gel content result. However, above 150 kGy, the ESR gels showed reduction in tensile value. This may be associated with the crosslinking and degradation that continuously take place in the ESR gels. The reduction in tensile value was due to the excessive crosslink density in the ESR gel. Ratnam and Zaman21 also reported similar observation on the electron beam-irradiated PVC/ENR blends. Compared to ESR, the irradiated EPDM showed a slight decline in tensile strength value, which is due to the same phenomenon that occurred in the ESR gels upon irradiation above 150 kGy.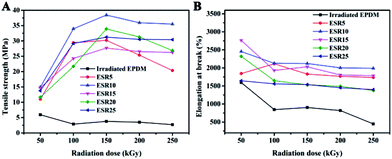 | ||
| Fig. 5 Change of (A) tensile strength and (B) elongation at break of ESR as a function of radiation dose. | ||
As shown in Fig. 5B, elongation at break decreases for irradiated EPDM and ESR gels as irradiation dose increases. Such reduction is expected since this property is found to decrease invariably upon irradiation regardless of whether chain scission or crosslinking is predominant. The acceleration in radiation-induced crosslinking increases with the increase of irradiation dose. This crosslinked network restricts the mobility of the molecular chains and causes the reduction in elongation at break.22,23 The relatively lower elongation at break value for irradiated EPDM is in perfect agreement with previous phenomenon as the gel fraction increases with decreasing t-BS content at the same irradiation dose.
3.3. Thermal properties of the ESR gels
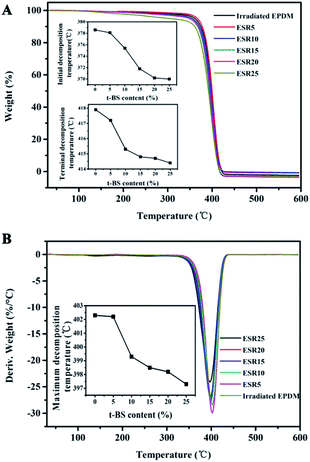
The effect of t-BS on the thermal weight-loss behavior of EPDM irradiated to 90 kGy was studied by means of TGA and DTG, the results were shown in Fig. 6. It was found that the initial and terminal decomposition temperatures of irradiated EPDM containing t-BS are lower than those of the unirradiated EPDM, and they slightly decreased with the increase of t-BS content due to the early degradation of t-BS (Fig. 6A). Furthermore, it should be noted that there are only one peak in the DTA curves of ESR, which demonstrates that the t-BS are distributed uniformly throughout EPDM matrix (Fig. 6B). Also, the maximum decomposition temperatures gradually decreased with an increase in t-BS content, which meant the graft of t-BS onto the EPDM has occurred definitely.3.4. Oil absorbent properties of the ESR gels
As mentioned above (Fig. 2B), the gel content of ESR gels increased as the radiation dose increased up to 200 kGy. However, when the gel fraction is lower than 80%, the samples lose their integrity before reaching equilibrium in oil solvents at room temperature. So, analysis of oil absorbency was carried out by the gel with fraction more than 80%. As shown in Fig. 7B–E, absorption curves have a similar trend when the t-BS content is less than 20 wt%. More specifically, as the irradiation dose increases, oil absorbency begins to decrease and then has a basically flat curve, which is attributed to the increase in crosslink density. Increase in irradiation dose will cause the formation of a denser network of the copolymer and reduce the chain length between cross-links which eventually reduces oil absorbency. When the t-BS content reached 25 wt%, the oil absorbency increased with an increase of irradiation dose to 75 kGy, but thereafter it decreased with any further increasing in irradiation dose (Fig. 7F). The initial increase in the maximum value of oil absorbency was probably due to the formation of complete network by increasing irradiation dose. The reason why the maximum value of oil absorbency decreased with further increase in irradiation dose was expected because the higher irradiation dose, the higher the crosslink degree of the network, resulting in a lower maximum value of oil absorbency.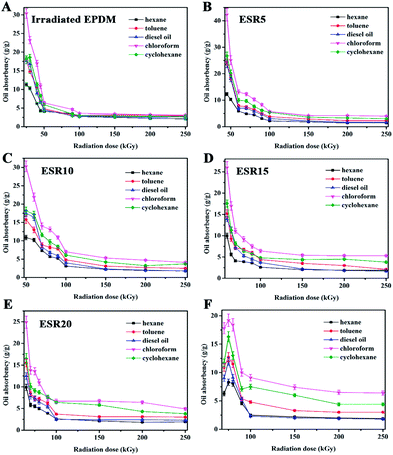 | ||
| Fig. 7 Oil absorbency of (A) irradiated EPDM, (B) ESR5, (C) ESR10, (D) ESR15, (E) ESR20 and (F) ESR25 in various oil-solvents as a function of radiation dose. | ||
It was also observed that the oil solvent absorbency of the ESR gels increased with the introduction of t-BS at the beginning, which could be explained by the fact that the 4-tert-butyl on t-BS occupies some space in the network. When t-BS content was 5 wt%, the oil absorbency reached maximum, and the maximum value of oil absorbency was 42.3 g g−1 in chloroform. This value is obviously larger compared to 30 times its weight for commercial oil sweep.24 When t-BS content surpasses 5 wt%, the oil absorbency decreases, which probably due to the formation of a denser network structure of ESR gel, since the addition of t-BS content can increase the polymeric gels rigidity caused by phenyl group in t-BS structure.
More importantly, it can be seen in the oil absorption curve that the oil absorption performance of the ESR can vary almost linearly within a small radiation dose range. This means that we can fine-tune the oil absorption of ESR by adjusting the radiation dose, making the final product can better meet the practical applications. Generally, it is difficult to form a kind of relaxing network with both a high gel fraction and low cross-link density via using common cross-link agents. The irradiation method not only provides a convenience approach to prepare oil-absorptive gels, but also offers significant possibilities for accurate control of oil-absorbency.
4. Conclusions
In conclusion, a high oil absorptive gel was successfully prepared by the use of an irradiation method as confirmed by infrared tests and gel fraction analysis. Gel content of the ESR gels was decreased with the increase of t-BS fraction; and the crosslinking density was found to increase with dose up to 90 kGy. This observation was attributed to that EPDM is more prone to radiation effect in comparison to t-BS, and predominately undergoes crosslinking. The tensile strength of ESR increased with increasing of irradiation dose up to 150 kGy, while elongation at break decreased with the radiation dose. Thermogravimetric measurement demonstrates that the ESR gel is homogenous and possesses good thermal stability. It was found that the optimum reaction conditions for obtaining the maximum oil absorbency of ESR gel were 5 wt% t-BS and a 50 kGy irradiation dose. The highest oil absorptivity of the ESR5 gel was 42.3 g g−1 in chloroform. Moreover, the oil-absorption test showed that almost linear change could be caused by a slightly change of radiation dose, which provided the possibility of precisely controlling the oil absorption of the final product. This finding opens up a new avenue for the design and preparation of controllable oil-absorptive gels through green and low operation cost irradiation method.Acknowledgements
This work was financially supported by state key laboratory of polymer materials and engineering of China (Grant No. sklpme2015-2-06).Notes and references
- H. X. Jin, B. Dong, B. Wu and M. H. Zhou, Polym.-Plast. Technol. Eng., 2012, 51, 154 CrossRef CAS.
- B. Wu, M. Zhou and D. Lu, Iran. Polym. J., 2006, 15, 989 CAS.
- S. Davis, W. Hellens and H. Zahalka, in Polymeric Materials Encyclopedia, ed. J. C. Salamone, CRC Press, New York, 1996, vol. 4, p. 2264 Search PubMed.
- K. G. Park, J. G. Park, C. S. Ha and W. J. Cho, J. Appl. Polym. Sci., 1999, 74, 3259 CrossRef CAS.
- M. H. Zhou and W. J. Cho, Polym. Int., 2001, 50, 1193 CrossRef CAS.
- M. H. Zhou and W. J. Cho, J. Appl. Polym. Sci., 2002, 85, 2119 CrossRef CAS.
- B. Wu and M. H. Zhou, Polym.-Plast. Technol. Eng., 2008, 47, 483 CrossRef CAS.
- B. Wu and M. H. Zhou, J. Appl. Polym. Sci., 2009, 112, 2213 CrossRef CAS.
- A. G. Chmielewski and M. Haji-Saeid, Radiat. Phys. Chem., 2004, 71, 17 CrossRef CAS.
- T. Yasin, S. Ahmed, M. Ahmed and F. Yoshii, Radiat. Phys. Chem., 2005, 73, 155 CrossRef CAS.
- Z. A. A. Sakinah, C. T. Ratnam, A. L. Chuah and T. C. S. Yaw, J. Elastomers Plast., 2011, 43, 239 CrossRef CAS.
- C. V. Chaudhari, Y. K. Bhardwaj, N. D. Patil, K. A. Dubey, V. Kumar and S. Sabharwal, Radiat. Phys. Chem., 2005, 72, 613 CrossRef CAS.
- K. A. Dubey, Y. K. Bhardwaj, C. V. Chaudhari and S. Sabharwal, J. Appl. Polym. Sci., 2006, 99, 3638 CrossRef CAS.
- J. Gehring, Radiat. Phys. Chem., 2000, 57, 361 CrossRef CAS.
- M.-H. Zhang, M.-M. Fan, C. Ma, H. Luo, P.-w. Wu and X. Zhang, RSC Adv., 2016, 6, 104934 RSC.
- V. Vijayabaskar and A. K. Bhowmick, J. Appl. Polym. Sci., 2005, 95, 435 CrossRef CAS.
- A. Rivaton, S. Cambon and J. L. Gardette, Nucl. Instrum. Methods Phys. Res., Sect. B, 2005, 227, 343 CrossRef CAS.
- A. Rivaton, S. Cambon and J. L. Gardette, Nucl. Instrum. Methods Phys. Res., Sect. B, 2005, 227, 357 CrossRef CAS.
- E. Richaud, X. Colin, B. Fayolle, L. Audouin and J. Verdu, Int. J. Chem. Kinet., 2008, 40, 769 CrossRef CAS.
- N. Khelidj, X. Colin, L. Audouin, J. Verdu, C. Monchy-Leroy and V. Prunier, Polym. Degrad. Stab., 2006, 91, 1593 CrossRef CAS.
- C. T. Ratnam and K. Zaman, Nucl. Instrum. Methods Phys. Res., Sect. B, 1999, 152, 335 CrossRef CAS.
- E. Planes, L. Chazeau, G. Vigier and J. Fournier, Polymer, 2009, 50, 4028 CrossRef CAS.
- E. Planes, L. Chazeau, G. Vigier, J.-M. Chenal and T. Stuhldreier, J. Polym. Sci., Part B: Polym. Phys., 2010, 48, 97 CrossRef CAS.
- D. c. website, Available from: http://www.dawginc.com.
| This journal is © The Royal Society of Chemistry 2017 |