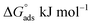Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of 1,2,3-triazoles in the presence of mixed Mg/Fe oxides and their evaluation as corrosion inhibitors of API 5L X70 steel submerged in HCl†
A. Espinoza-Vázquez a,
F. J. Rodríguez-Gómez
a,
F. J. Rodríguez-Gómez a,
B. I. Vergara-Arenas
a,
B. I. Vergara-Arenas b,
L. Lomas-Romero
b,
L. Lomas-Romero b,
D. Angeles-Beltrán
b,
D. Angeles-Beltrán c,
G. E. Negrón-Silva
c,
G. E. Negrón-Silva *c and
J. A. Morales-Serna
*c and
J. A. Morales-Serna b
b
aFacultad de Química, Departamento de Ingeniería Metalúrgica, Universidad Nacional Autónoma de México, Av. Universidad No. 3000, Coyoacán, C.U., Ciudad de México, C.P. 04510, Mexico
bDepartamento de Química, Universidad Autónoma Metropolitana-Iztapalapa, Av. San Rafael Atlixco No. 186, Ciudad de México, C.P. 09340, Mexico
cDepartamento de Ciencias Básicas, Universidad Autónoma Metropolitana-Azcapotzalco, Av. San Pablo No. 180, Ciudad de México, C.P. 02200, Mexico. E-mail: gns@correo.azc.uam.mx
First published on 9th May 2017
Abstract
In this work, the catalytic capacity of a Mg/Fe layered double hydroxide (LDH) and its mixed oxides to perform Huisgen cycloaddition was studied. The catalytic process was performed in the presence of sodium ascorbate, which was determinant for the success of the 1,3-cycloaddition. The obtained triazoles were evaluated as corrosion inhibitors using electrochemical impedance spectroscopy under static conditions, showing an inhibition efficiency higher than 95% at 50 ppm for some of the triazoles studied. According to the Langmuir isotherm, all the compounds synthesized and analysed exhibit a chemisorption process. Finally, the corrosion process when submerging a steel bar in 1 M HCl was studied using SEM-EDS. This experiment showed that the corrosion process decreases considerably in the presence of 50 ppm of the organic inhibitor.
1. Introduction
One of the main problems in heavy industry is the corrosion of metallic materials, which is directly reflected in economic losses for this productive sector.1 Usually, the industrial removal of iron oxides is performed using mineral acids, of which the most used is hydrochloric acid. However, their aggressiveness accelerates the degradation of materials, causing them to dissolve in acid medium.2Currently, the oil industry has resorted to organic inhibitors containing N, O and S in their basal structure to counteract the corrosion problem.3 These types of inhibitors are obtained efficiently using easy-access synthesis pathways and are usually used at lower concentrations during the corrosion inhibition process.4
Among this group of organic molecules, we find triazoles, with 1,2,4-triazoles being the most used for this purpose.5 However, special attention has been recently focused on 1,2,3-triazoles as inhibitors due to the ease with which they are synthetically obtained and their notable efficiency as inhibitors.6 In this context, this paper reports the synthesis of 1-benzyl-4-phenyl-1,2,3-triazoles, as well as the evaluation of their capacity to inhibit corrosion in an acid aqueous medium. The study focuses on the analysis of aromatic systems substituted with electron donor groups (Fig. 1), which have not been previously studied for such applications.
Another important point to be highlighted in this study is the catalytic system used to obtain the triazoles, which are commonly synthesized in the presence of catalytic systems containing Cu(II) or Cu(I).7 In this case, the synthesis of triazoles was performed in the presence of a Mg/Fe layered double hydroxide (LDH) (Mg/Fe hydrotalcite) and their mixed oxides. Although the Mg/Fe LDH has been used in several organic transformations, it has not been studied as a catalyst in a Huisgen reaction to obtain triazoles.7
2. Experimental
2.1. API 5L X70 steel
API 5L X72 type steel was used, which has a metallographic preparation with the following composition nominal (wt%): C, 0.025; Mn, 1.65; Si, 0.26; Ti, 0.015; V, 0.001; Nb, 0.068; Mo, 0.175; S, 0.0025; Al, 0.045; Ni, 0.08; Cr, 0.07; Cu, 0.21 and Fe, balance.82.2. Solution preparation
A 0.01 M solution of the triazoles 1a–1h in DMF was prepared (Fig. 1). Then, concentrations of 5, 10, 20 and 50 ppm of the inhibitor were added to the 1 M HCl corrosive solution using a Gill AC device.2.3. Electrochemical evaluation
The potential was stabilized at 20 °C for approximately 1800 s before electrochemical impedance spectroscopy (EIS) test.EIS: a sinusoidal potential of ±20 mV was applied in a frequency interval of 10−1 Hz to 104 Hz, in an electrochemical cell with three electrodes. The working electrode was API 5L X70 steel, the reference electrode was saturated Ag/AgCl, and the counter electrode was graphite. The electrode surface was prepared using conventional metallography methods on an exposed area of 4.52 cm2.
After EIS measurements, the potentiodynamic polarization curves of 5 and 50 ppm of inhibitors were obtained. The measurements covered a range of −500 mV to 500 mV regarding the open circuit potential (OCP), with a sweep velocity of 66.07 mV min−1 using the ACM Analysis software for data interpretation.
2.4. Characterization of surfaces by SEM-EDS
The API 5L X70 steel surface was prepared both without (blank) and with inhibitor; a 50 ppm concentration was used for a 24 h immersion time. After that experiment, the steel was washed with distilled water, dried and the surface analysed using a Zeiss SUPRA 55 VP electronic sweep microscope at 10 kV with a 300× secondary electron detector.3. Results and discussion
3.1. Synthesis of 1,2,3-triazoles
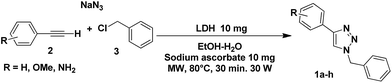
The Mg/Fe LDH was synthesized and characterized following the steps described in the literature.9 Then, a comparative study was performed on the use of the synthesized and calcined LDH on the multi-component reaction to obtain the 1-benzyl-4-phenyl-1,2,3-triazoles, from alkyne 2, benzyl chloride 3 and sodium azide, in the presence or absence of sodium ascorbate with an ethanol–water mixture as solvent. The best reaction yields for each alkyne were obtained when the catalytic process was performed with calcined LDH in the presence of sodium ascorbate (Table 1). If the heterogeneous catalyst was not calcined, the yield decreased considerably (Table 1). This result is a consequence of the larger surface area of the calcined material (mixed oxides) compared with the non-calcined material (LDH).| Entry | Triazole | Yieldb (%) | |
|---|---|---|---|
| LDH dry | LDH calcined | ||
a Reagents: alkyne 2 (1 mmol), benzyl chloride 3 (1.2 mmol), NaN3 (1.2 mmol), catalyst (10 mg) and EtOH–H20 (2 mL, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).b Yield of isolated product after chromatographic purification.c The reaction was performed in the absence of benzyl chloride.d The reaction was performed in the presence of sodium ascorbate (10 mg).e The reaction was performed in the absence of sodium ascorbate. 1).b Yield of isolated product after chromatographic purification.c The reaction was performed in the absence of benzyl chloride.d The reaction was performed in the presence of sodium ascorbate (10 mg).e The reaction was performed in the absence of sodium ascorbate. |
|||
| 1c | 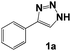 |
40d | 60d |
| 15e | 30e | ||
| 2 | 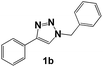 |
48d | 65d |
| 15e | 30e | ||
| 3 | 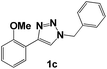 |
40d | 55d |
| 10e | 35e | ||
| 4 | 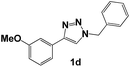 |
40d | 55d |
| 10e | 30e | ||
| 5 | 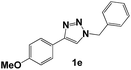 |
45d | 60d |
| 10e | 30e | ||
| 6 | 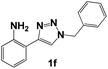 |
30d | 50d |
| 10e | 25e | ||
| 7 | 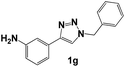 |
25d | 5d |
| 5e | 25e | ||
| 8 | 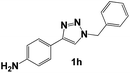 |
25d | 55d |
| 10e | 30e | ||
It was also observed that the presence of sodium ascorbate was determinant for obtaining excellent yields in the cycloaddition process (Table 1). This result can be explained if we consider that the sodium ascorbate sequesters Fe(II) from the material to reduce it to Fe(I), which is more active during the catalytic process. In this way, the global catalytic process involves a heterogeneous catalyst (LDH or Mg/Fe mixed oxides) containing Fe(II) and a homogeneous catalyst with Fe(I) associated with the ascorbate.
The above agrees with descriptions in the literature for the same organic reaction using similar catalytic systems (LDH and Cu/Al mixed oxides).10 However, the main contribution of this work is the use of an LDH containing Fe(II) or its mixed oxides, which had never previously been used as the catalyst in a cycloaddition reaction of a terminal alkyne and a derived azide. Another important point to highlight is that the catalyst can be recovered and reused for three reaction cycles, with the same efficiency as previously described. Then, the material loses efficiency, and the yield of the organic reaction decreases considerably.
Once the triazoles 1a–1h were synthesized, they were evaluated as corrosion inhibitors, as structurally they represent a different triazole family from the ones previously reported in the literature. In this case, the electron donor substituents are present in the aromatic ring from the alkyne (Fig. 1), in contrast to models that were previously studied in our research group, where the substituents were found in the benzylic aromatic ring.6
3.2. Electrochemical evaluation
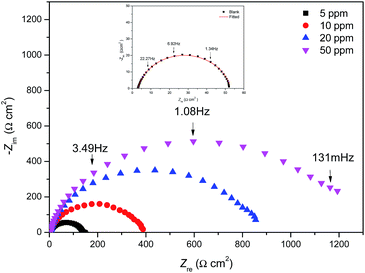
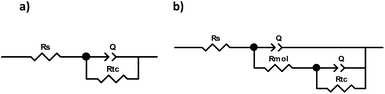
The Nyquist diagram (Fig. 2) corresponds to the system without inhibitor (blank) and the different concentrations of compound 1a. It can be observed that in the absence of the inhibitor, the semicircle formed shows a time constant reaching only a Zre value of 50 Ω cm2. Moreover, for all concentrations studied, a depressed circle is observed, denoting that the dissolution process is controlled by the charge transference resistance.11 The deformation in the semicircles of the different concentrations is due to the material's ruggedness, the active sites or the non-homogeneity of the solids.12 However, it shows a continuous increase in the Zre value.Then, using the equivalent electrical circuits, the impedance diagrams shown in Fig. 3 were adjusted to obtain the corresponding resistances and thus to calculate the inhibition efficiency. Fig. 3a is used when the system has no inhibitor, and Fig. 3b corresponds to the system when different concentrations of the inhibitor have been added.
The efficiency of the proposed inhibitors (ηEIS%) can be calculated using the following equation:
 | (1) |
Table 2 shows the results obtained after fitting the experimental data with the equivalent electrical circuits of Fig. 3. It can be observed that the Rct increases as the inhibitor concentration increases, reaching a maximum at 50 ppm with an inhibition efficiency of 96.1%, allowing triazoles 1a to be considered an excellent corrosion inhibitor. On the other hand, the capacitance value of the double electrochemical layer (Cdl) decreases due to the gradual displacement of water molecules with the compound 1a molecules in the working electrode, which decreases the number of active sites and consequently delays the corrosion phenomenon.13
| C/ppm | 0 | 5 | 10 | 20 | 50 |
| Rs/Ω cm2 | 5.0 | 5.3 | 5.1 | 5.1 | 5.1 |
| ±SD | 0.00 | 0.02 | 0.01 | 0.01 | 0.01 |
| n | 0.8 | 0.9 | 0.8 | 0.9 | 0.9 |
| Cdl/μF cm−2 | 1490.0 | 147.9 | 147.2 | 147.1 | 143.6 |
| ±SD | 0.0 | 23.3 | 53.4 | 64.2 | 39.3 |
| Rct/Ω cm2 | 50.0 | 167.3 | 437.3 | 919.9 | 1285.3 |
| ±SD | 0.0 | 23.3 | 53.4 | 64.2 | 39.3 |
| ηEIS/% | — | 69.5 | 88.4 | 94.5 | 96.1 |
| ±SD | — | 4.4 | 1.4 | 0.4 | 0.1 |
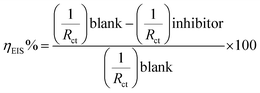
After demonstrating the efficiency of compound 1a, triazole 1b, which has a benzyl group in position 1 of the triazole ring, was studied. Fig. 4a shows the Nyquist diagram corresponding to triazole 1b at different concentrations, with a continuous increase in the Zre value reaching a maximum of ∼2000 Ω cm2 at 50 ppm. By comparing the Nyquist diagrams of inhibitors 1a and 1b, it can be observed that from 5 ppm, the Zre value is higher for inhibitor 1b, reaching ∼500 Ω cm2, which is attributed to the presence of the benzyl group at position 1 of the triazole ring.
 | ||
| Fig. 4 Nyquist diagrams for different concentrations of (a) 1b, (b) 1c, (c) 1d and (d) 1e on API 5L X70 submerged in 1 M HCl. | ||
Then, we examined the effect of the electron donor groups in one of the aromatic rings (Fig. 1, compounds 1c–1h). First, we considered the three isomers (ortho 1c, meta 1d and para 1e) containing a methoxide group (–OCH3). The Nyquist diagrams for these three isomers are shown in Fig. 4.
Compound 1c shows a continuous increase in the Zre value, which is controlled by the charge transference resistance (Fig. 4b).14
The diameters of the semicircles are lower than for the compound without the methoxide group 1b, which is attributed to the presence of oxygen with pairs of free, unshared electrons. These pairs of electrons can be located in a p orbital parallel to the p orbitals forming the aromatic system, which allows the generation of resonant structures with high electronic density in the aromatic ring.
The Nyquist diagram for triazoles 1d and 1e (Fig. 4c and d) shows the sweep of the different concentrations analysed. In both cases, two time constants can be observed, one attributed to the charge transference resistance and the other to the inhibitor's molecules.15 Likewise, it can be observed that the Zre value does not show large variation. Based on these results, it can be inferred that the presence of a methoxide group in the ortho, meta and para positions of the aromatic ring is not involved in the increase of the inhibition capacity, based on comparison to triazole 1b.
Table 3 shows the different results obtained after fitting to the experimental data, revealing that the corrosion inhibition increases when the inhibitor concentration increases. In contrast, the presence of a methoxide group in the chemical structure decreases the charge transfer resistance value, and therefore, the corrosion inhibition decreases.
| Triazole | C/ppm | Rs/Ω cm2 | ±SD | n | Cdl/μF cm−2 | ±SD | Rct/Ω cm2 | ±SD | ηEIS/% | ±SD |
|---|---|---|---|---|---|---|---|---|---|---|
| a SD = standard deviation of at least three measurements. | ||||||||||
| 1b | 0 | 5 | 0 | 0.8 | 1490 | 0 | 50 | 0 | ||
| 5 | 4.3 | 0.0 | 0.9 | 112.4 | 5.9 | 683.7 | 126.9 | 92.4 | 1.5 | |
| 10 | 4.1 | 0.0 | 0.9 | 107.8 | 1.8 | 1350.0 | 66.4 | 96.3 | 0.2 | |
| 20 | 4.1 | 0.0 | 0.9 | 110.0 | 3.8 | 1693.0 | 101.4 | 97.0 | 0.2 | |
| 50 | 4.2 | 0.0 | 0.9 | 116.9 | 1.9 | 2306.0 | 174.4 | 97.8 | 0.2 | |
| 1c | 5 | 4.2 | 0.0 | 0.8 | 119.7 | 9.9 | 416.7 | 56.9 | 87.8 | 1.7 |
| 10 | 4.2 | 0.0 | 0.9 | 125.8 | 4.3 | 638.9 | 42.6 | 92.1 | 0.5 | |
| 20 | 4.2 | 0.0 | 0.8 | 127.4 | 3.3 | 820.6 | 29.8 | 93.9 | 0.2 | |
| 50 | 4.2 | 0.0 | 0.8 | 129.6 | 4.5 | 1046.8 | 68.4 | 95.2 | 0.3 | |
| 1d | 5 | 1.6 | 0.4 | 0.8 | 106.6 | 13.3 | 420.5 | 33.8 | 88.0 | 0.9 |
| 10 | 1.7 | 0.3 | 0.7 | 103.3 | 21.1 | 421.1 | 26.0 | 88.1 | 0.8 | |
| 20 | 2.4 | 0.5 | 0.7 | 112.9 | 12.9 | 471.5 | 14.0 | 89.4 | 0.3 | |
| 50 | 3.0 | 0.3 | 0.7 | 117.0 | 11.9 | 498.5 | 5.9 | 90.0 | 0.1 | |
| 1e | 5 | 0.6 | 0.0 | 0.6 | 273.4 | 34.5 | 131.2 | 14.0 | 73.3 | 2.2 |
| 10 | 0.7 | 0.2 | 0.9 | 309.1 | 157.7 | 240.4 | 19.6 | 79.1 | 1.8 | |
| 20 | 0.8 | 0.1 | 0.9 | 391.4 | 157.8 | 248.7 | 77.2 | 78.0 | 6.3 | |
| 50 | 0.9 | 0.1 | 0.9 | 369.5 | 88.0 | 237.0 | 13.9 | 78.8 | 1.2 | |
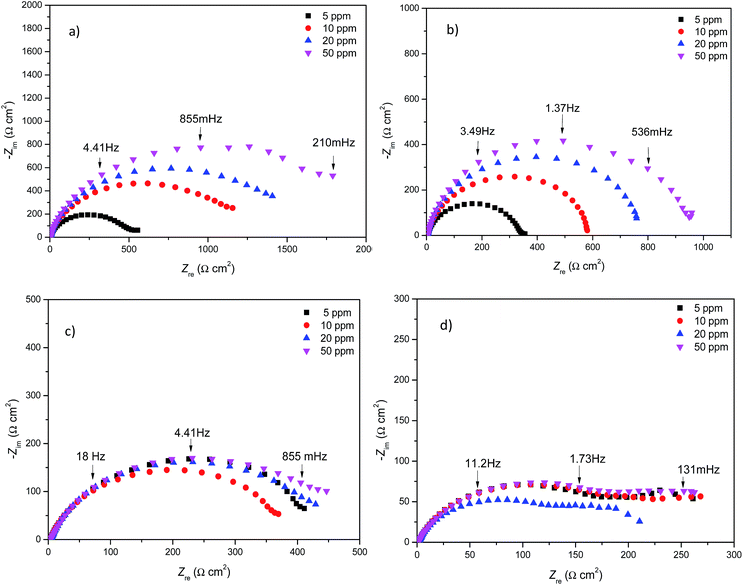
Next, the 1,2,3-triazoles where the methoxide group (–OMe) was substituted with an amine (–NH2) were evaluated. The Nyquist diagrams for triazoles 1f, 1g and 1h are shown in Fig. 5. All these diagrams show an increase in the Zre value as the inhibitor concentration increases. Based on these diagrams, it can be deduced that the amine group (–NH2) in the ortho position favourably influences the corrosion process (Fig. 5).
 | ||
| Fig. 5 Nyquist diagrams for different concentrations of (a) 1f, (b) 1g and (c) 1h on API 5L X70 submerged in 1 M HCl. | ||
Again, after the experimental data fitting, Table 4 shows the electrochemical parameters for triazoles 1f, 1g and 1h. The Rct values increase as the inhibitor concentration increases, in contrast to what happens to the Cdl values, which decrease. This result is attributed to the displacement of water molecules by the inhibitor molecules.16 On the other hand, the second time constant proposed in the Nyquist diagrams is attributed to the resistance of organic molecules (Rmol) for compounds 1f and 1g.
| Triazole | C/ppm | Rs/Ω cm2 | ±SD | n | Cdl/μF cm−2 | ±SD | Rct/Ω cm2 | ±SD | Rmol/Ω cm2 | ±SD | ηEIS/% | ±SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1f | 0 | 5.0 | 0.0 | 0.8 | 1490.0 | 0.0 | 50.0 | 0.0 | 0.0 | — | ||
| 5 | 10.7 | 0.1 | 0.9 | 66.7 | 19.1 | 419.4 | 210.4 | 117.7 | 91.3 | 80.5 | 5.2 | |
| 10 | 10.7 | 0.2 | 0.9 | 72.3 | 17.6 | 1937.0 | 131.1 | 170.1 | 85.1 | 97.4 | 0.2 | |
| 20 | 5.3 | 0.1 | 0.9 | 138.8 | 26.8 | 1076.2 | 100.5 | 205.9 | 10.2 | 95.3 | 0.4 | |
| 50 | 5.3 | 0.1 | 0.9 | 143.4 | 72.3 | 1545.0 | 28.3 | 115.9 | 8.6 | 96.8 | 0.1 | |
| 1g | 5 | 7.3 | 0.0 | 0.9 | 187.2 | 45.2 | 336.9 | 93.1 | 26.0 | 13.2 | 83.6 | 5.5 |
| 10 | 7.4 | 0.0 | 0.9 | 170.0 | 12.6 | 376.7 | 88.6 | 95.6 | 76.2 | 85.9 | 3.4 | |
| 20 | 6.7 | 0.5 | 0.9 | 163.3 | 5.2 | 473.7 | 23.0 | 1.0 | 0.2 | 89.4 | 0.5 | |
| 50 | 7.0 | 0.0 | 0.9 | 167.1 | 1.7 | 460.2 | 36.9 | 3.2 | 3.0 | 89.1 | 0.8 | |
| 1h | 5 | 1.6 | 0.0 | 0.7 | 336.7 | 11.7 | 164.5 | 3.6 | — | — | 69.6 | 0.7 |
| 10 | 1.7 | 0.1 | 0.7 | 283.2 | 16.3 | 220.8 | 11.0 | — | — | 77.3 | 1.1 | |
| 20 | 1.8 | 0.0 | 0.7 | 296.5 | 18.7 | 238.8 | 11.8 | — | — | 79.0 | 1.0 | |
| 50 | 1.7 | 0.1 | 0.7 | 331.2 | 17.1 | 237.9 | 1.9 | — | — | 79.0 | 0.2 |
Fig. 6 shows the comparison of the different 1,2,3-triazoles. The presence of the benzyl group at position 1 of the triazole ring is determinant for obtaining excellent inhibition results with compound 1b (η ∼ 96.8% at 50 ppm, Fig. 6a). The presence of methoxide or amine electron donor groups at the meta or para position in one of the aromatic rings does not have a notable impact on the efficiency of the system when inhibiting corrosion (Fig. 6b and c). However, electron donor groups at the ortho position to influence the corrosion inhibition process; the best inhibitors are 1c with η ∼ 93.6% and 1f with η ∼ 96.8% at 50 ppm.
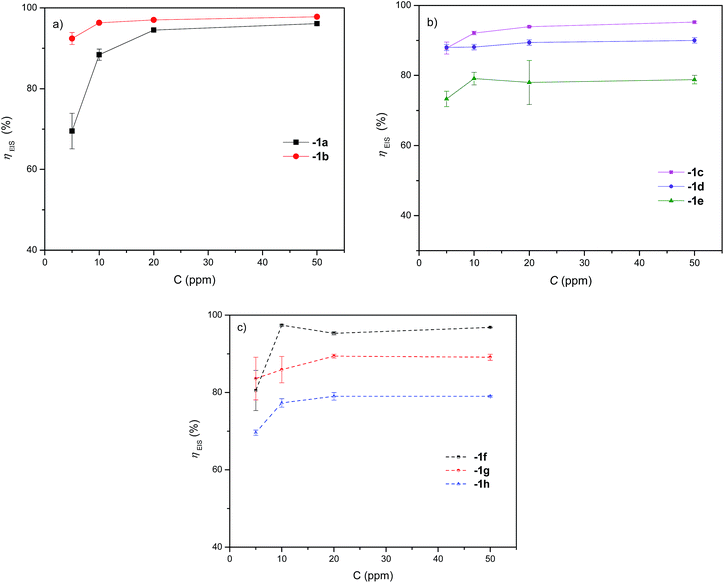 | ||
| Fig. 6 Variation of the inhibition efficiency by EIS technique of 1a, 1b and derivatives 1c–1h as a function of API 5L X70 steel concentration submerged in 1 M HCl. | ||
3.3. Polarization curves
Tafel polarization curves of API 5L X70 steel at 5 and 50 ppm of triazoles are shown in Fig. 7. The corrosion potential (Ecorr), corrosion current density (icorr), Tafel anodic slopes (βa), Tafel cathodic slopes (βc), and inhibition efficiency (η), are shown in Table 5.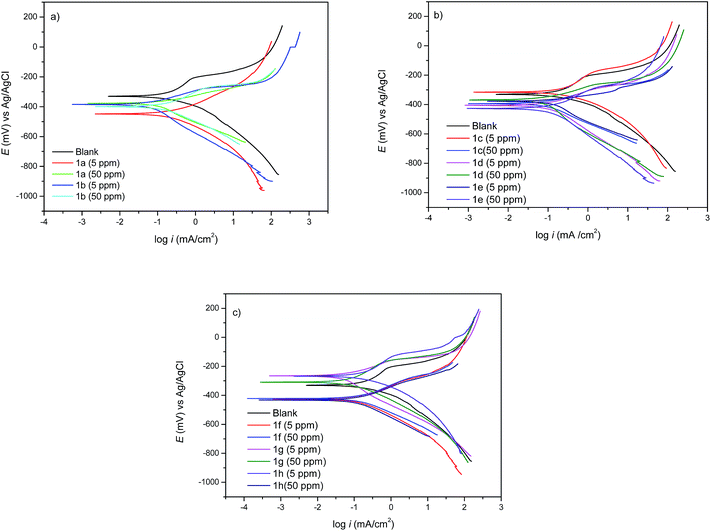 | ||
| Fig. 7 Potentiodynamic polarization curves at different 1,2,3-triazoles derivatives concentrations for API 5L X70 steel submerged in 1 M HCl. | ||
| Inhibitor | C/ppm | Ecorr/mV vs. Ag/AgCl | βa/mV dec−1 | βc/mV dec−1 | icorr/mA cm−2 | ηpol/% |
|---|---|---|---|---|---|---|
| BLANK | — | −330.97 | 173.4 | 125.1 | 0.5303 | — |
| 1a | 5 | −428.7 | 84.5 | 132.9 | 0.1320 | 75.1 |
| 50 | −377.5 | 99.0 | 36.4 | 0.0518 | 90.2 | |
| 1b | 5 | −385.7 | 156.0 | 52.1 | 0.0561 | 89.4 |
| 50 | −373.7 | 111.0 | 59.2 | 0.0602 | 88.7 | |
| 1c | 5 | −385.0 | 155.0 | 63.8 | 0.0841 | 84.1 |
| 50 | −393.9 | 111.0 | 76.8 | 0.0640 | 87.9 | |
| 1d | 5 | −405.4 | 184.1 | 79.9 | 0.1159 | 78.1 |
| 50 | −371.2 | 215.1 | 65.5 | 0.0743 | 86.0 | |
| 1e | 5 | −377.7 | 118.6 | 35.9 | 0.0688 | 87.0 |
| 50 | −425.0 | 195.8 | 129.2 | 0.1160 | 78.1 | |
| 1f | 5 | −421.0 | 113.0 | 88.0 | 0.1354 | 74.5 |
| 50 | −264.5 | 142.4 | 50.9 | 0.0338 | 93.6 | |
| 1g | 5 | −265.4 | 141.5 | 67.5 | 0.0336 | 93.7 |
| 50 | −309.3 | 127.7 | 88.1 | 0.1050 | 80.2 | |
| 1h | 5 | −432.1 | 127.6 | 95.0 | 0.1127 | 78.7 |
| 50 | −472.0 | 113.5 | 137.1 | 0.1035 | 80.5 |
The inhibition efficiency of the organic compounds (triazoles) were calculated by:
 | (2) |
In Fig. 7a–c for triazoles derivatives under static conditions, it can be observed how the current density decreases in their presence, which suggests that it is retarding the corrosion process due to the protective film formed by the inhibitor on the metallic surface.17
The results shown in Table 5 correspond to the corrosion potential (Ecorr) in the presence of the inhibitor 1b, 1c, 1d and 1g. The difference between the values of blank and the inhibitors is less than 85 mV, which suggests18 that triazoles show a mixed predominantly anodic type inhibition. On the other hand, inhibitors 1f and 1h show a cathodic type behaviour.
3.4. Adsorption process
After calculating the electrochemical parameters for the different organic compounds under static conditions, it is necessary to describe the interaction of the inhibitor with the metallic surface, calculating the surface coverage (θ) using the eqn (3) employing the inhibition efficiency (η) from eqn (1).19
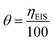 | (3) |
In the literature, there are several adsorption models relating the coating degree to the metallic surface; including the Temkin, Freundlich, Frumkin20 or Langmuir models.21 The latter was used in this study. Once the fitting was performed with the Langmuir adsorption model (eqn (4)), it was observed that it possesses a linear fit, given that it obtained a correlation coefficient (R2) close to 1.
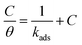 | (4) |
Fig. 8a–c show the Langmuir isotherm model for each of the corrosion inhibitors, showing that the 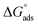 value of organic compounds 1b–1e is higher than −40 kJ mol−1 (Table 6). These values, according to the literature reports,22 represent a chemisorption interaction of the inhibitor with the metallic surface. It is noteworthy that the inhibitor 1a exhibits a
value of organic compounds 1b–1e is higher than −40 kJ mol−1 (Table 6). These values, according to the literature reports,22 represent a chemisorption interaction of the inhibitor with the metallic surface. It is noteworthy that the inhibitor 1a exhibits a  between −20 kJ mol−1 and −40 kJ mol−1, which indicates the simultaneous physical and chemical adsorption of this molecule on mild steel surface immersed in HCl.
between −20 kJ mol−1 and −40 kJ mol−1, which indicates the simultaneous physical and chemical adsorption of this molecule on mild steel surface immersed in HCl.
 | ||
| Fig. 8 Langmuir isotherm of triazoles 1a–1h as corrosion inhibitors of the surface of API 5L X70 submerged in 1 M HCl. | ||
3.5. SEM-EDS
Fig. 9 shows the microscopy pictures and the corresponding chemical analysis for the polished metal, in the presence and absence of the inhibitor, to confirm the efficiency of each compound obtained by the EIS. The polished steel consists of Fe, Mn, C and Si (Fig. 9a). When the metal was submerged in hydrochloric acid, it was observed that in addition to the steel composition, O and Cl are present as a consequence of the corrosion phenomenon (Fig. 9b). In the microscopy pictures corresponding to samples with the inhibitor (Fig. 9c–e), a decrease in corrosion can be observed.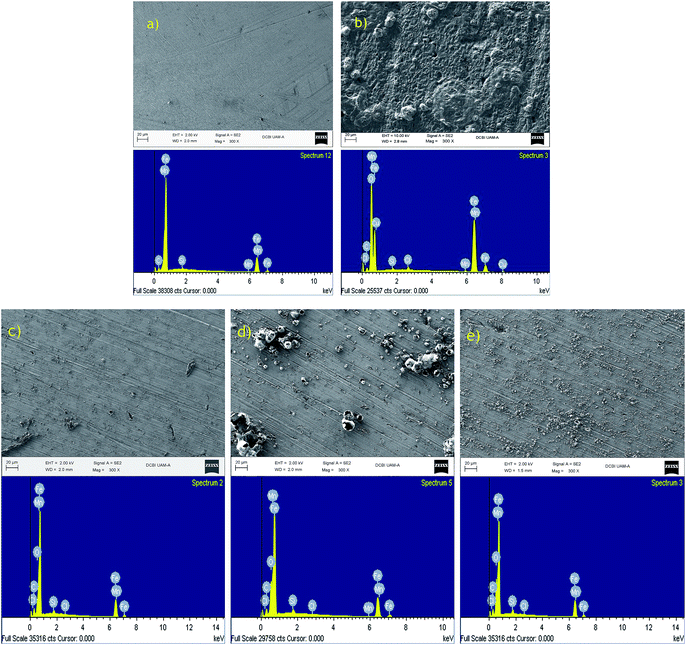 | ||
| Fig. 9 SEM-EDX spectra of API 5L X70 steel: (a) polished steel, (b) in presence of corroding medium, and in presence of 50 ppm (c) 1b, (d) 1c and (e) 1f. | ||
To determine the elements present in API 5L X70 steel surface after 5 hours of exposure to 1 M HCl, EDX analysis were used. Before the corrosion test, the peaks are related to the elements present in the API 5L X70 steel (Table 7). In the absence of inhibitors, the spectra exhibit the peaks of oxygen and chloride which regarding to polished API 5L X70 do not have. The spectra of API 5L X70 steel immersed in 1 M HCl containing 50 ppm of 1b, 1c and 1f inhibitors show that the amount of oxygen and chloride decreases, probably due to the formation of a triazole derivative film on the surface of steel.
| Element | % atomic | ||||
|---|---|---|---|---|---|
| Steel | Steel + HCl | 1b | 1c | 1f | |
| C | 14.21 | 9.51 | 15.84 | 11.49 | 12.57 |
| O | — | 51.62 | 7.09 | 16.11 | 19.31 |
| Si | 0.45 | 0.3 | 1.23 | 1.19 | 1.2 |
| Cl | — | 0.67 | 0.1 | — | 0.24 |
| Mn | 0.82 | 0.31 | — | — | — |
| Fe | 84.5 | 37.59 | 92.53 | 71.21 | 66.67 |
3.6. Inhibition mechanism
In the inhibition mechanism two main types of interaction exist that can describe the adsorption of the inhibitors: physisorption and chemisorption. Several authors describe the inhibition mechanism by protonated species in acid medium (Scheme 1a), which are attracted toward the solid/liquid interface to form a protective film, preventing the metal from interacting with the aggressive medium (physisorption).23 On the other hand, the adsorption of 1,2,3-triazoles can also occur due to the interactions between the d-orbital of iron atoms (coordinate type bond), with the lone sp2 electron pairs present on the heteroatoms of the inhibitor (chemisorption, Scheme 1b). In this case, both models can help to explain the behaviour of triazoles as corrosion inhibitors.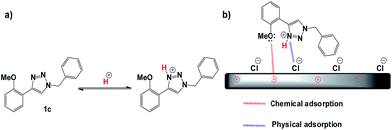 | ||
| Scheme 1 (a) Protonation and (b) schematic adsorption model of the triazole 1c on API 5L X70 surface in 1 M HCl. | ||
4. Conclusions
The synthesis of 1,2,3-triazoles is usually performed in the presence of Cu(I) and Cu(II) salts. However, in this study, we demonstrated that a solid catalytic material such as a Mg(II)/Fe(III) hydrotalcite and its mixed oxides can catalyse the Huisgen cycloaddition with good yields. After the catalytic process, it is possible to recover the heterogeneous catalyst for reuse in two additional reaction cycles with the same efficiency.On the other hand, the 1-benzyl-4-phenyl-1,2,3-triazoles obtained were evaluated as corrosion inhibitors, demonstrating that the presence of activator groups in the ortho position of one of the aromatic rings in the inhibitor system influences the corrosion inhibition process of API 5L X70 steel submerged in 1 M HCl. The adsorption of the studied compounds, using the Langmuir equation, reveals that the inhibitor molecule blocks the active sites by a chemisorption process.
Acknowledgements
The authors would like to thank Consejo Nacional de Ciencia y Tecnología (CONACyT project-239938) for financial support. AEV is grateful to DGAPA-UNAM for the Postdoctoral Grant. FJRG is grateful to FQ-UNAM for the PAIP support. JAMS acknowledges support by the Royal Society (UK), Newton Fellowship Alumni program.Notes and references
- D. A. Winkier, M. Breedon, P. White, A. E. Hughes, E. D. Sapper and I. Cole, Corros. Sci., 2016, 106, 229 CrossRef.
- (a) K. Wan, P. Feng, B. Hou and Y. Li, RSC Adv., 2016, 6, 77515 RSC; (b) S. M. Shaban, RSC Adv., 2016, 6, 39784 RSC.
- (a) E. Garcia-Ochoa, S. J. Guzmán-Jiméz, J. G. Hernández, T. Pandiyan, J. M. Vásquez-Pérez and J. Cruz-Borbolla, J. Mol. Struct., 2016, 1119, 314 CrossRef CAS; (b) M. A. Hegazy, S. S. Abd El Rehim, A. M. Badawi and M. Y. Ahmed, RSC Adv., 2015, 5, 49070 RSC; (c) M. A. Hegazy, J. Mol. Liq., 2015, 208, 227 CrossRef CAS; (d) H. Tian, Y. Frank Cheng, W. Li and B. Hou, Corros. Sci., 2015, 100, 341 CrossRef CAS; (e) M. S. Shihab and A. F. Mahmood, Russ. J. Appl. Chem., 2016, 89, 505 CrossRef CAS; (f) A. L. Chong, J. I. Mardel, D. R. MacFarlane, M. Forsyth and A. E. Somers, ACS Sustainable Chem. Eng., 2016, 4, 1746 CrossRef CAS; (g) M. H. Keshavarz, K. Esmaelpour, A. N. Golikand and Z. Shirazi, Z. Anorg. Allg. Chem., 2016, 642, 906 CrossRef CAS.
- (a) S. K. Saha, A. Dutta, P. Ghosh, D. Sukul and P. Banerjee, Phys. Chem. Chem. Phys., 2016, 18, 17898 RSC; (b) M. Shabani, N. M. Behpour, F. Sadat, R. M. Hamadanianb and V. Nejadshafiee, RSC Adv., 2015, 5, 23357 RSC.
- (a) M. El Bakri, R. Touir, N. Dkhireche, M. Ehn Touhami, A. Rochdi and A. Zarrouk, Arabian J. Sci. Eng., 2016, 41, 75 CrossRef CAS; (b) D. K. Ivanou, K. A. Yasakau, S. Kallip, A. D. Lisenkov, M. Starkevich, S. V. Lamaka, M. G. S. Ferreira and M. L. Zheludkevich, RSC Adv., 2016, 6, 12553 RSC; (c) G. Rajkunar and M. G. Sethuraman, Res. Chem. Intermed., 2016, 42, 1809 CrossRef; (d) J. Balaji and M. G. Sethuraman, Res. Chem. Intermed., 2016, 42, 1328 CrossRef.
- (a) A. Espinoza-Vázquez, F. J. Rodriguez-Gómez, R. González-Olvera, D. Angeles-Beltran, D. Mendoza-Espinosa and G. E. Negrón-Silva, RSC Adv., 2016, 18, 72885 RSC; (b) A. Espinoza-Vázquez, G. E. Negrón-Silva, R. González-Olvera, D. Angeles-Beltran, M. Romero-Romo and M. Palomar-Pardavé, Arabian J. Sci. Eng., 2017, 42, 163 CrossRef; (c) A. Espinoza-Vázquez, G. E. Negrón-Silva, R. González-Olvera, D. Angeles-Beltran, H. Herrera-Hernández, M. A. Romero-Romo and M. E. Palomar-Pardavé, Mater. Chem. Phys., 2014, 145, 407 CrossRef.
- For recent reviews about synthesis of 1,2,3-triazole, see: (a) H. B. Jalani, A. Ç. Karagöz and S. B. Tsogoeva, Synthesis, 2017, 49, 29 CAS; (b) C. Wang, D. Ikhlef, S. Kahlal and J.-Y. Saillard, Coord. Chem. Rev., 2016, 316, 1 CrossRef CAS; (c) S. Chassaing, V. Bénéteau and P. Pale, Catal. Sci. Technol., 2016, 6, 923 RSC; (d) V. Castro, H. Rodríguez and F. Albericio, ACS Comb. Sci., 2016, 18, 1 CrossRef CAS PubMed; (e) Z.-J. Zheng, D. Wang, Z. Xu and L.-W. Xu, Beilstein J. Org. Chem., 2015, 11, 2557 CrossRef CAS PubMed; (f) A. Laurina, R. Delisi, F. Mingoia, A. Terenzi, A. Martorana, G. Barone and M. Almerico, Eur. J. Org. Chem., 2014, 3289 CrossRef.
- M. A. Mohtadi-Bonab, J. A. Szpunar, R. Basu and M. Eskandari, Int. J. Hydrogen Energy, 2015, 40, 1096 CrossRef CAS.
- (a) K. Morimoto, K. Tamura, H. Yamada, T. Sato and M. Suzuki, Appl. Clay Sci., 2016, 121–122, 71 CrossRef CAS; (b) A. Dias, L. Cunha and A. C. Vieira, Mater. Res. Bull., 2011, 46, 1346 CrossRef CAS; (c) N. B.-H. Abdelkader, A. Bentouami, Z. Derriche, N. Bettahar and L.-C. de Ménorval, Chem. Eng. J., 2011, 169, 231 CrossRef; (d) W. Meng, F. Li, D. G. Evans and X. Duan, Mater. Res. Bull., 2004, 39, 1185 CrossRef CAS; (e) J. Das, D. Das, G. P. Dash and K. M. Parida, J. Colloid Interface Sci., 2002, 251, 26 CrossRef CAS PubMed.
- R. González-Olvera, C. I. Urquiza-Castro, G. E. Negrón-Silva, D. Ángeles-Beltrán, L. Lomas-Romero, A. Gutiérrez-Carrillo, V. H. Lara, R. Santillán and J. A. Morales-Serna, RSC Adv., 2016, 6, 63660 RSC.
- (a) K. Kermannezhad, A. C. Najafi, M. M. Mohsen and B. Rezae, Chem. Eng. J., 2016, 306, 849 CrossRef CAS; (b) P. Singh, V. Srivastava and M. A. Quraishi, J. Mol. Liq., 2016, 216, 164 CrossRef CAS; (c) K. H. Hassan, A. A. Khadom and N. H. Kurshed, S. Afr. J. Chem. Eng., 2016, 22, 1 Search PubMed.
- A. Khadiri, R. Saddik, K. Bekkouche, A. Aouniti, B. Hammouti, N. Benchat, M. Bouachrine and R. Solmaz, J. Taiwan Inst. Chem. Eng., 2016, 58, 552 CrossRef CAS.
- (a) C. Verma, M. A. Quraishi and A. Singh, Journal of Taibah University for Science, 2016, 10, 718 CrossRef; (b) F. Xu, J. Duan, S. Zhang and B. Hou, Mater. Lett., 2008, 62, 4072 CrossRef CAS.
- (a) F. Bentiss, C. Jama, B. Mernari, H. E. Attari, L. E. Kadi, M. Lebrini, M. Traisnel and M. Lagrenée, Corros. Sci., 2009, 51, 1628 CrossRef CAS; (b) H.-L. Wang, R.-B. Liu and J. Xin, Corros. Sci., 2004, 46, 2455 CrossRef CAS.
- (a) A. Döner, R. Solmaz, M. Özcan and G. Kardaş, Corros. Sci., 2011, 53, 2902 CrossRef; (b) M. A. Hegazy, M. Abdallah, M. K. Awad and M. Rezk, Corros. Sci., 2014, 81, 54 CrossRef CAS; (c) S. John and A. Joseph, Mater. Chem. Phys., 2012, 133, 1083 CrossRef CAS.
- (a) A. Y. Musa, A. A. H. Kadhum, A. M. Bakar and M. T. Sobri, Corros. Sci., 2010, 52, 3331 CrossRef CAS; (b) D. M. Gurudatt, K. N. Mohana and H. C. Tandon, Materials Discovery, 2015, 2, 24 CrossRef.
- N. Kumar, C. Verma, M. A. Quraishi and A. K. Mulherjee, J. Mol. Liq., 2016, 215, 47 CrossRef.
- S. Shaban, A. Abd-Elaal and S. Tawfik, J. Mol. Liq., 2016, 216, 392 CrossRef CAS.
- (a) Z. Tao, W. He, S. Wang, S. Zhang and G. Zhou, Corros. Sci., 2012, 60, 205 CrossRef CAS; (b) Z. Hu, Y. Meng, X. Ma, H. Zhu, J. Li, C. Li and D. Cao, Corros. Sci., 2016, 112, 563 CrossRef CAS.
- (a) C. Verma, M. A. Quraishi and A. Singh, J. Mol. Liq., 2015, 212, 804 CrossRef CAS; (b) P. Singh, M. J. Makowska, P. Slovensky and M. A. Quraishi, J. Mol. Liq., 2016, 220, 71 CrossRef CAS; (c) M. S. Salih and A.-D. H. Hussien, J. Mol. Struct., 2014, 1076, 658 CrossRef; (d) T. C. Krishnamurthy, K. N. M. Shetty and H. T. Chander, J. Mol. Liq., 2015, 211, 1026 CrossRef.
- (a) M. Yadav, S. Kumar, R. R. Sinha, I. Bahadur and E. E. Ebenso, J. Mol. Liq., 2015, 211, 135 CrossRef CAS; (b) A. Yurt, B. Duran and H. Dal, Arabian J. Chem., 2014, 7, 732 CrossRef CAS.
- (a) N. Yilmaz, A. Fitoz, Ü. Ergun and K. C. Emregül, Corros. Sci., 2016, 111, 110 CrossRef CAS; (b) M. I. Awad, J. Appl. Electrochem., 2006, 36, 1163 CrossRef CAS; (c) H. Jafari, Trans. Indian Inst. Met., 2016, 69, 805 CrossRef CAS; (d) A. Teimouri, N. Soltani and A. N. Chermahini, Front. Chem. Sci. Eng., 2011, 5, 43 CrossRef CAS.
- (a) G. Karthik, M. Sundaravadivelu and P. Rajkumar, Res. Chem. Intermed., 2015, 41, 1543 CrossRef CAS; (b) M. Farsak, H. Keles and M. Keles, Corros. Sci., 2015, 98, 223 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra01325f |
| This journal is © The Royal Society of Chemistry 2017 |