A novel approach for quantitative LIBS fluorine analysis using CaF emission in calcium-free samples
C.
Alvarez-Llamas
,
J.
Pisonero
 and
N.
Bordel
*
and
N.
Bordel
*
Department of Physics, University of Oviedo, c/Gonzalo Gutiérrez Quirós, Campus de Mieres, 33600 Mieres, Spain. E-mail: bordel@uniovi.es
First published on 2nd December 2016
Abstract
The determination of fluorine in solid samples using Laser Induced Breakdown Spectroscopy (LIBS) is a challenging task due to the low excitation efficiency of this element. Recently, the use of molecular CaF bands was demonstrated to improve the analytical capabilities of LIBS fluorine detection in Ca-containing samples. In this work, a novel approach has been developed to extend this methodology for fluorine quantification in calcium-free samples. In particular, the on-line nebulization of a Ca-containing solution on the surface of a fluorine containing sample has been successfully evaluated to obtain the desired CaF molecular emission. Nebulization parameters have been optimized in order to maximize the molecular emission. A linear relationship between the CaF molecular emission signal and the amount of F in the solid samples has been obtained. The calculated limit of detection for fluorine (about 50 μg g−1) is in the same order of magnitude as that obtained for Ca-containing samples. This novel approach for fluorine quantification opens new ways for the analysis of halogens in solid samples.
Introduction
Laser-Induced Breakdown Spectroscopy (LIBS) is an analytical technique based on the use of a short duration laser pulse that is focused on the surface of a sample, generating a laser-induced plasma where different atomization, ionization, recombination and excitation processes take place. The radiation emitted by the excited species present in the plasma provides useful chemical and physical information about the sample. Some of the most cited advantages of LIBS include reduced analysis time, minimal to no sample pre-treatment, multi-elemental detection, high spatial resolution and the potential to carry out in-lab, in situ or standoff analysis.1,2 However, the determination of halogen species, in particular fluorine and chlorine, is not an easy task due to the high energy of the excited electronic levels (e.g. the most intense emission lines for these elements lie in the VUV region). At these wavelengths (e.g. <100 nm in the case of fluorine3), the whole experimental set-up has to be operated under vacuum conditions to avoid the absorption of the emitted radiation from the atmospheric compounds. The measurement of the lower intensity F I infrared emission lines in the spectral range between 680 and 780 nm is a practical alternative.4–10 In particular, the use of a helium atmosphere in the surrounding environment of the laser-induced plasma, which increases the signal-to-background ratio of these F I atomic emission lines, is a recommended option for fluorine analysis by LIBS.The presence of molecular emission bands in the laser-induced plasma spectra has been observed since the early stages of the LIBS technique,11 and it could be attributed to the recombination process of the plasma species when the plasma cools down. Recently it was probed that the use of CaF molecular emission is a good alternative to the use of a helium atmosphere for fluorine quantitative studies in Ca containing samples,12–14 especially in the low μg g−1 fluorine concentration range.15 This strategy was originally proposed during the first half of the 20th century to be used in arc spectroscopy as an alternative to the direct measurement of F emission lines.16–18
In this context, the fluorine quantification of calcium-free samples is a challenging task. Therefore, in this work, a novel approach to achieve CaF emission based on the online external spraying of a calcium solution on the surface of fluorine-containing samples is investigated. In particular, a fast, easy and versatile methodology, based on the use of a total consumption nebulizer is developed and optimized to determine traces of fluorine via CaF molecular emission in Ca-free samples.
Experimental
The LIBS experimental set-up used in this work is based on a Q-switched Nd:YAG laser (NL301HT from EKSPLA, Vilnius, Lithuania) at 1064 nm. The laser pulse energy was fixed at 100 mJ per pulse using an attenuator (LOTIS-TII, Minsk, Belarus), and the shot repetition rate was selected as 10 Hz. The laser beam was focused on the sample surface using an objective with a focal length of 35 mm (LMH-5X-1064 from Thorlabs, Newton, NJ, USA). Samples were moved using motorized X–Y micro-translation stages. The laser-induced plasma radiation was recorded using a combination of plane-convex lenses, placed at 45° with respect to the sample surface plane and coupled to an optical fiber. The other end of the optical fiber was fixed to the entrance slit of a Czerny–Turner spectrometer (Shamrock SR-500i-D1 from Andor Technology, Belfast, Northern Ireland). The spectrometer has a focal length of 500 mm and a grating of 1200 lines per mm. Emission signals were finally recorded using an iCCD camera (Andor iStar).The samples employed in this work were prepared by mixing NaF (purity > 99.5%) and Cu powder (purity > 99.7%). These samples were selected in order to use the same type of samples employed in a previous study,15 but in the present study the samples do not contain Ca. A pre-homogenization step was performed by mixing the powdered samples with methanol, shaking and drying. The mixed powdered samples were then deposited and distributed over a double-sided tape, fixed on a glass microscope slide.
A raster pattern composed of fourteen lines, with a length of 15 mm and a separation of 1 mm, was measured in each powder sample. After each raster line, a LIBS spectrum is obtained by accumulating 10 single shot emission spectra. Then the fourteen spectra are averaged to obtain the final spectrum. Additionally, three replicates were measured using this protocol for each sample. Data treatment was carried out using the “LA/LIBS data analysis software” by Applied Spectra, Inc. Fremont, CA, USA.
The calcium nebulization process was performed using a total consumption nebulizer (DS-5 Microflow Concentric Nebulizer; Teledyne CETAC Technologies, Omaha, 68144, USA), placed near the laser induced plasma. The Ca solution (calcium nitrite solution (30% w/w) in water (Sigma-Aldrich, Merk, Darmstadt, Germany)) was introduced using a syringe pump (Thermo Fisher Scientific, Waltham, USA), with a gas flow of 1 l min−1 of argon (introduced using a mass flow controller from MKS Instruments, Andover, USA). Fig. 1a and b show a schematic view of the experimental set-up and a photo of the nebulizer, including the sample stage and the objective arrangement. The nebulizer was placed at an angle of 50° with respect to the sample plane.
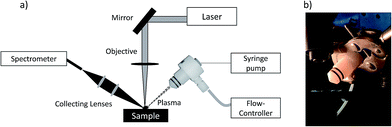 | ||
| Fig. 1 a) Schematic diagram of the LIBS experimental set-up; b) Picture of the nebulization process during the analysis. | ||
Results and discussion
Proof of concept: Teflon sample
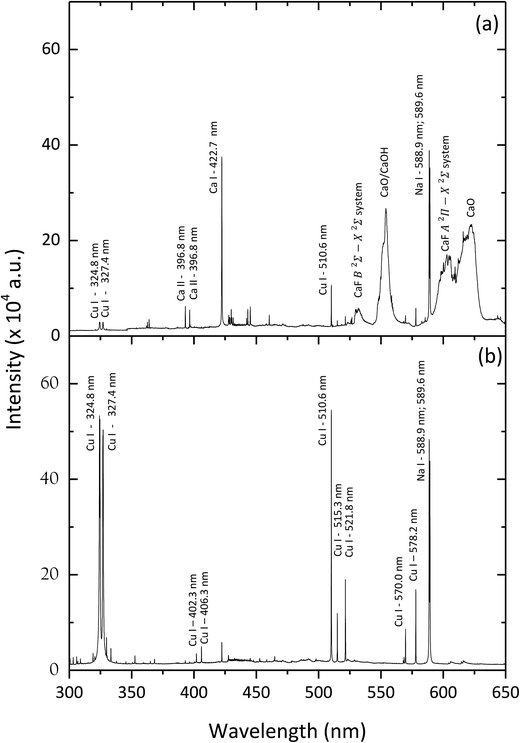
The most intense molecular system for CaF emission in LIBS is the “Orange system” A2Π − X2Σ; however, this system was discarded due to the presence of strong interference of CaO at the same spectral region. Therefore, the CaF molecular emission bands of the denominated “Green system” B2Σ − X2Σ were used as the analytical signal in this study. The most prominent emission bands of this molecular system lie between 529 and 542 nm.The formation of these CaF molecules in Ca-free samples during the nebulization of a Ca nitrite solution was investigated. In particular, a polytetrafluoroethylene sample (Teflon) was employed to evaluate this novel methodology. LIBS operating conditions were optimized for molecular detection (e.g. a delay time of 20 μs and a gate width of 50 μs). The intensity of the CaF molecular emission bands enhanced at increasing concentrations of calcium nitrite in the nebulized solution. In particular, a solution with 15% of calcium nitrite was employed, since higher concentrations start to produce the obstruction of the capillary tube in the nebulizer. Lower calcium nitrite concentrations might also be used but it would affect the sensitivity of the CaF analytical signal. The influence of the nebulization flow path was evaluated in order to obtain the maximum signal for the CaF molecular emission. Fig. 2 shows the LIBS emission spectra from a Teflon sample, obtained before and during the nebulization of the Ca solution on the sample surface. It is observed that the LIBS emission spectrum from the raw Teflon does not show any CaF molecular signal (only the presence of the C2 Swam system at 516 nm is observed in this spectral interval). However, when the Ca solution is nebulized towards the sample surface, an intense CaF molecular signal is detected. The emergence of the CaF molecular emission is attributed to the recombination of F ablated from the Teflon sample with Ca present at the sample surroundings and with Ca deposited on the sample surface and also ablated by the laser shot.
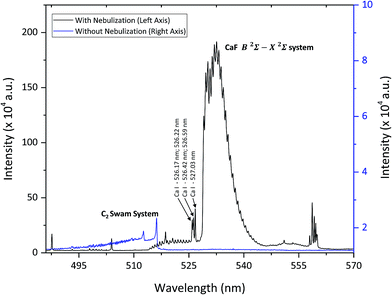 | ||
| Fig. 2 LIBS emission spectra from a Teflon sample, before Ca nebulization on the sample (blue line, right axis) and during nebulization (black line, left axis). | ||
Application to samples containing F traces
Once the formation of CaF by nebulization of a Ca solution on a F-containing sample during the ablation process is verified, the methodology must be optimized for calcium-free samples containing traces of fluorine. Samples selected for this next step were powdered samples containing fluorine in a Cu matrix.In a previous study with similar samples, it was established that a minimum Ca![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
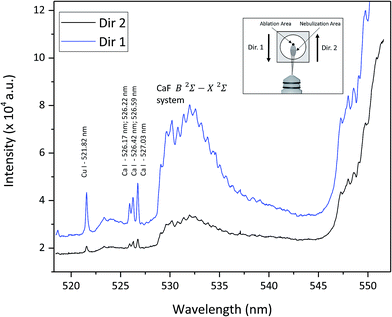
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F molar ratio of 20 was required to obtain an analytical response from the CaF molecular emission independent of the Ca content.15 In this work, the flow of the Ca solution in the total consumption nebulizer also needs to be optimized to achieve the proper formation of the CaF molecule. This nebulizer scatters small amounts of solution (a few μl min−1); however, the presence of small droplets of water over the sample surface is known to produce plasma quenching effects. Therefore, the influence of the Ca nebulization on the LIBS signal was investigated. Fig. 3 shows the emission spectra of a powdered Cu sample (mass content: 99.8% of Cu and 1500 μg g−1 of F) obtained with and without Ca nebulization. The formation of the CaF and CaO molecular bands is clearly observed when the nebulization process takes place. In addition, atomic and ionic Ca emission lines appear in this spectrum while the intensity of atomic Cu emission lines diminishes in comparison with those measured in the spectrum obtained with no nebulization. Spectra acquisition is carried out by moving the sample while consecutive laser impacts produce a raster pattern in the sample. Therefore, the direction of the sample translation, against the nebulization beam or in the same direction, can play an important role in the resulting spectra. Fig. 4 shows the LIBS emission spectra of the powdered Cu sample (with 1500 μg g−1 of F) detected during Ca nebulization, moving the sample along two opposite directions with respect to the nebulization flow. According to the schematic inset showed in Fig. 4, in each laser shot, the laser beam is focused at the edge of the area that is getting wet by the nebulization flow. Therefore, when the sample is moved in the same direction as the nebulization flow, the ablation is taking place in sample regions with a higher amount of water content than when the sample is moved in the opposite direction. From Fig. 4 it is inferred that the sample should be translated along the direction of the nebulization flow to avoid the ablation of highly humidified surface regions, where the emission signal is significantly attenuated.
F molar ratio of 20 was required to obtain an analytical response from the CaF molecular emission independent of the Ca content.15 In this work, the flow of the Ca solution in the total consumption nebulizer also needs to be optimized to achieve the proper formation of the CaF molecule. This nebulizer scatters small amounts of solution (a few μl min−1); however, the presence of small droplets of water over the sample surface is known to produce plasma quenching effects. Therefore, the influence of the Ca nebulization on the LIBS signal was investigated. Fig. 3 shows the emission spectra of a powdered Cu sample (mass content: 99.8% of Cu and 1500 μg g−1 of F) obtained with and without Ca nebulization. The formation of the CaF and CaO molecular bands is clearly observed when the nebulization process takes place. In addition, atomic and ionic Ca emission lines appear in this spectrum while the intensity of atomic Cu emission lines diminishes in comparison with those measured in the spectrum obtained with no nebulization. Spectra acquisition is carried out by moving the sample while consecutive laser impacts produce a raster pattern in the sample. Therefore, the direction of the sample translation, against the nebulization beam or in the same direction, can play an important role in the resulting spectra. Fig. 4 shows the LIBS emission spectra of the powdered Cu sample (with 1500 μg g−1 of F) detected during Ca nebulization, moving the sample along two opposite directions with respect to the nebulization flow. According to the schematic inset showed in Fig. 4, in each laser shot, the laser beam is focused at the edge of the area that is getting wet by the nebulization flow. Therefore, when the sample is moved in the same direction as the nebulization flow, the ablation is taking place in sample regions with a higher amount of water content than when the sample is moved in the opposite direction. From Fig. 4 it is inferred that the sample should be translated along the direction of the nebulization flow to avoid the ablation of highly humidified surface regions, where the emission signal is significantly attenuated.
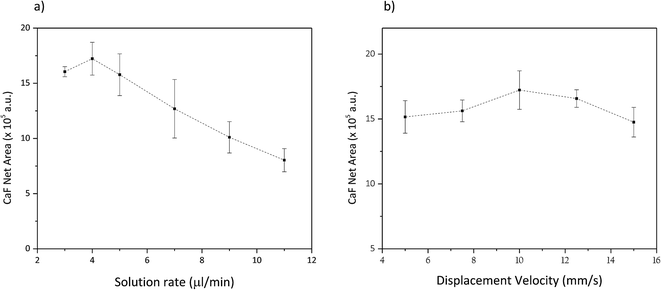
The optimum amount of Ca-solution was also studied, considering the flow rate in the nebulizer and the sample displacement velocity. Fig. 5a shows the variation of the net signal from the CaF Green System emission bands (integrated between 528 and 543 nm) as a function of the Ca solution flow rate nebulized over the surface of the Cu powdered sample. It is observed that in the evaluated flow rate interval (between 3 and 11 μl min−1) the net emission signal shows a relative maximum around 4 μl min−1. It must be highlighted that a higher flow rate is related not only to a higher amount of Ca but also to humidity on the surface of the sample. Fig. 5b shows the variation of the net emission signal as a function of the displacement velocity of the sample along the axis of the nebulizer. It is noticed that in the investigated range (between 5 and 15 mm s−1) the displacement velocity does not show significant influence on the emission signal. Therefore, an average displacement velocity of 10 mm s−1 was selected for the further experiments.
Trace fluorine quantitative analysis
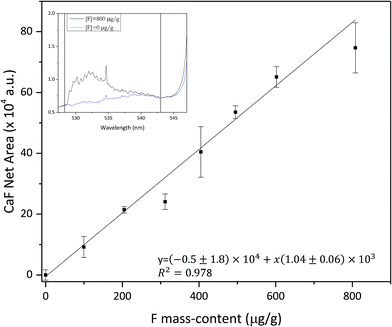
Under the optimised conditions (a Ca solution flow rate of 4 μl min−1 and a displacement velocity of 10 mm s−1), several Ca-free samples with increasing fluorine content (in a concentration range between 100 and 800 μg g−1) were analysed during the nebulization of the Ca solution. The temporal conditions of LIBS acquisition were optimised looking for a balance between the higher CaF molecular signal and the reduction of the atomic emission signals (e.g. Ca I at 534.95 nm), that decay at an earlier time domain in the laser induced plasma. Therefore, temporal conditions were fixed at a delay time of 35 μs and a gate width of 70 μs. Fig. 6 collects the variation of the CaF net emission signal versus the fluorine mass content. It is observed that this calibration curve shows a linear response. Uncertainties were calculated as the standard deviation from three sample replicates. The variation in the value of some uncertainties might come from the sample preparation steps (e.g. inhomogeneous deposition on the double-sided tape, or a heterogeneous distribution of the powdered sample). The inset graph in Fig. 6 shows the emission spectra of a sample with a F content of 800 μg g−1 of , and of a blank sample (0 μg g−1 of fluorine) collected during Ca solution nebulization. The analytical signal used in the calibration curve corresponds to the net area of the molecular emission bands between the vertical straight lines at 528 and 543 nm. The net area for each sample was calculated by the subtraction of the blank signal to the corresponding spectrum. It has to be noted that the contribution of the emission line at 534.95 nm to the analytical signal was also subtracted. From this calibration curve, a detection limit of 49 μg g−1 of fluorine mass-content was estimated using the 3σ criteria, improving those achieved using other LIBS approaches for the detection of fluorine. This result points out that this analytical method, based on the nebulization of a Ca solution close to the sample surface during LIBS analysis, is an interesting and promising approach for LIBS fluorine quantification in solid samples, providing good detection limits.Conclusions
In the present work a novel methodology for fluorine quantification, using LIBS emission from molecular CaF bands in calcium free samples, has been developed. The absence of calcium in the sample has been satisfactorily overcome by the nebulization of a calcium containing solution over the sample surface (e.g. calcium nitrite solution in water). Different nebulization operating parameters have been investigated and optimized. In particular, it has been shown that the presence of highly humidified sample surface regions could limit the analytical capabilities of this method due to a decrease in the intensity of the atomic emission lines in the recorded spectra.This methodology has been successfully applied to the quantitative determination of fluorine in Cu powdered samples. A linear calibration curve was obtained using samples with a known fluorine mass content, resulting in a detection limit of 49 μg g−1 of fluorine mass-content. It is important to notice that the developed methodology has the potential to be used, not only in the case of fluorine analysis, but also in the analysis of other halogen elements, such as chlorine, whose molecular emission is found to be stronger than the atomic one.
Acknowledgements
The authors would like to acknowledge the financial support from the Government of the Principality of Asturias through the project FC-15-GRUPIN14-040 and from the Ministerio de Economia (Spain), through the national project MINECO-13-CTQ2013-49032-C2-2-R.References
- D. W. Hahn and N. Omenetto, Appl. Spectrosc., 2010, 64, 335–366 CrossRef PubMed.
- D. Cremers and L. J. Radziemski, Handbook of Laser-Induced Breakdown Spectroscopy, John Wiley & Sons Ltd, Oxford, UK, 1st edn, 2013 Search PubMed.
- J. E. Sansonetti, W. C. Martin and S. L. Young, Handbook of Basic Atomic Spectroscopic Data, 2005, https://www.nist.gov/pml/handbook-basic-atomic-spectroscopic-data (Accessed October 2016) Search PubMed.
- M. Tran, B. W. Smith, D. W. Hahn and J. D. Winefordner, Appl. Spectrosc., 2001, 55, 1455–1461 CrossRef CAS.
- D. Cremers and L. J. Radziemski, Anal. Chem., 1983, 55, 1252–1256 CrossRef CAS.
- M. Tran, Q. Sun, B. W. Smith and J. D. Winefordner, Appl. Spectrosc., 2001, 55, 739–744 CrossRef CAS.
- L. St-Onge, E. Kwong, M. Sabsabi and E. Vadas, Spectrochim. Acta, Part B, 2002, 57, 1131–1140 CrossRef.
- C. D. Quarles, J. J. Gonzalez, L. J. East, J. H. Yoo, M. Morey and R. E. Russo, J. Anal. At. Spectrom., 2014, 29, 1238 RSC.
- G. Asimellis, S. Hamilton, A. Giannoudakos and M. Kompitsas, Spectrochim. Acta, Part B, 2005, 60, 1132–1139 CrossRef.
- C. González de Vega, C. Álvarez Llamas, N. Bordel, R. Pereiro and A. Sanz-Medel, Anal. Chim. Acta, 2015, 877, 33–40 CrossRef PubMed.
- J. Debras-Guédon and N. Liodec, C. R. Hebd. Seances Acad. Sci., 1963, 257, 3336–3339 Search PubMed.
- M. Gaft, L. Nagli, N. Eliezer, Y. Groisman and O. Forni, Spectrochim. Acta, Part B, 2014, 98, 39–47 CrossRef CAS.
- C. Álvarez Llamas, J. Pisonero and N. Bordel, Spectrochim. Acta, Part B, 2014, 100, 123–128 CrossRef.
- O. Forni, M. Gaft, M. J. Toplis, S. M. Clegg, S. Maurice, R. C. Wiens, N. Mangold, O. Gasnault, V. Sautter, S. Le Mouélic, P.-Y. Meslin, M. Nachon, R. E. McInroy, A. M. Ollila, A. Cousin, J. C. Bridges, N. L. Lanza and M. D. Dyar, Geophys. Res. Lett., 2015, 42, 1020–1028 CrossRef CAS.
- C. Alvarez-Llamas, J. Pisonero and N. Bordel, Spectrochim. Acta, Part B, 2016, 123, 157–162 CrossRef CAS.
- J. Papish, L. E. Hoag and W. E. Snee, Ind. Eng. Chem., Anal. Ed., 1930, 2, 263–264 CrossRef CAS.
- H. W. Jaffe, Am. Mineral., 1948, 34, 667–673 Search PubMed.
- O. B. Troshkina, J. Appl. Spectrosc., 1971, 14, 430–437 CrossRef.
| This journal is © The Royal Society of Chemistry 2017 |