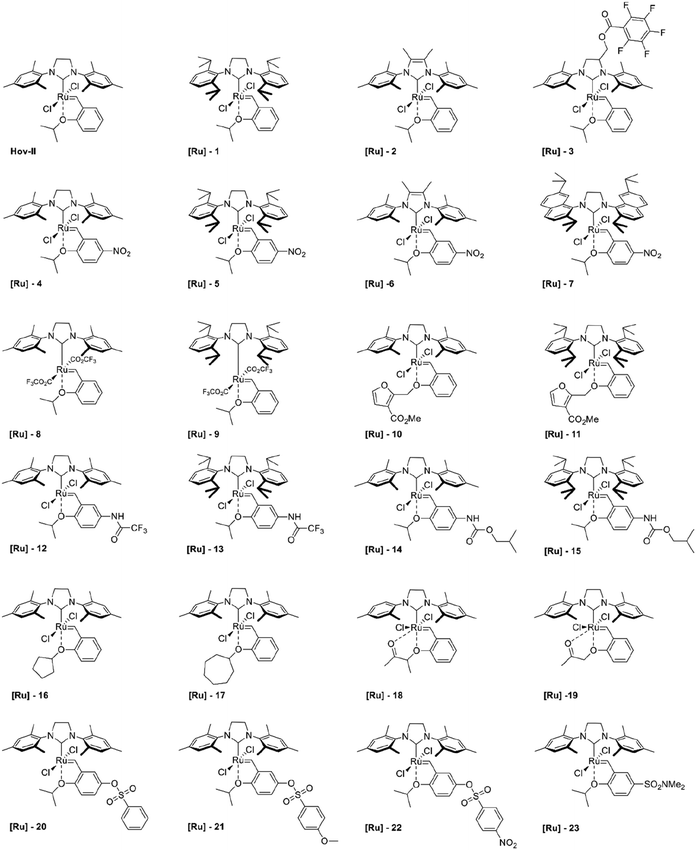
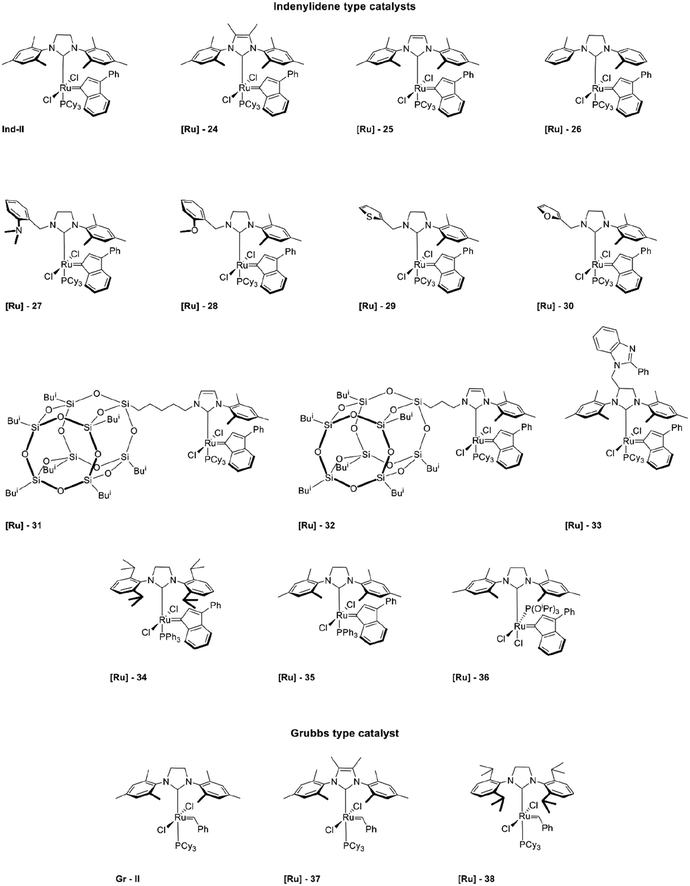
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fishing for the right catalyst for the cross-metathesis reaction of methyl oleate with 2-methyl-2-butene†
A.
Sytniczuk
a,
A.
Kajetanowicz
*b and
K.
Grela
 *a
*a
aFaculty of Chemistry, Biological and Chemical Research Centre, University of Warsaw, Żwirki i Wigury 101, 02-089 Warsaw, Poland. E-mail: prof.grela@gmail.com
bInstitute of Organic Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland. E-mail: anna.kajetanowicz@gmail.com
First published on 13th February 2017
Abstract
The activity of various Ru-alkylidene olefin metathesis catalyst types on the outcome of cross-metathesis of methyl oleate with 2-methyl-2-butene was studied.
1. Introduction
With the discovery of well-defined ruthenium catalysts, olefin metathesis has become an irreplaceable tool in the synthesis of carbon–carbon double bonds.1,2 Ring closing metathesis (RCM)3,4 and ring opening metathesis polymerisation (ROMP)5–7 are widely used in the large scale production of new materials,8,9 complex polymeric compounds,10,11 and various natural products, such as macrocyclic lactones with musk odor12 or antibiotics.13 Nonetheless, the greatest potential for the synthesis of the new compounds is related to utilization of the cross-metathesis (CM) reaction. Recent developments leading to metathesis catalysts with increased stability1,2 and thus greater tolerance to functional groups enabled easy functionalization of organic compounds.1,2 It has been proved that CM proceeds well not only with the unfunctionalised olefins but also with alkenes containing various functional groups such as vinyl sulfones,14 acrylonitrile,15,16 acrylic esters,17,18 or even vinyl halides,19,20 which were believed to be quite demanding substrates.Agenda 21, the comprehensive economic plan adopted by more than 180 governments, is encouraging the use of renewable natural resources21 like, inter alia, oils and fats of both plant and animal origin. The total market based on these valuable resources amounts to more than 140 million tons per year which is 5% of the total biomass produced every year. These oleochemicals are used for the production of important product groups22,23 such as surfactants,24,25 lubricants,25,26 and coatings,27 or even oil based paints, printing inks, and synthetic resins.
Plant oils and fats, due to their diverse construction and multiplicity of double bonds, are perfect substrates to various organic transformations such as oxidation,28 epoxidation,29 or transition-metal catalysed addition.30 As previously proved,31 olefin metathesis can also be a perfect tool to convert plant oils into valuable commodity chemicals; however, in the context of industrial use of this methodology the appropriate choice of the catalyst is crucial in order to achieve the most economical process characterised by the highest selectivity and conversion possible.32–37 Following the studies on acetate functionalisation,35–37 in the present article the influence of N-heterocyclic carbenes (NHCs), phosphorous ligands and alkylidene moieties present in the Hoveyda–Grubbs, Grubbs and indenylidene type ruthenium catalysts on cross-metathesis of methyl oleate with 2-methyl-2-butene was studied.
The CM reaction of olefins with an excess of 2-methyl-2-butene was described for the first time by Grubbs38 and can be seen as an effective and direct method for the conversion of a terminal mono-substituted C–C double bond into a trisubstituted “prenyl-type” one (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 → –CH
CH2 → –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMe2). Such a formed trisubstituted prenyl double bond undergoes a number of useful synthetic transformations, including electrophilic addition39 as well as recently intensively studied metal- and organo-catalysed reactions.40–44 In addition, the prenyl fragment has been shown to be important for protein–protein binding through specialized prenyl-binding domains.45
CMe2). Such a formed trisubstituted prenyl double bond undergoes a number of useful synthetic transformations, including electrophilic addition39 as well as recently intensively studied metal- and organo-catalysed reactions.40–44 In addition, the prenyl fragment has been shown to be important for protein–protein binding through specialized prenyl-binding domains.45
Although it was observed that the CM reaction with 2-methyl-2-butene is sometimes less selective38,46 than the analogous transformation with 2-methyl-propene47 the use of higher boiling 2-methyl-2-butene is more convenient from the practical point of view than working with gaseous and more expensive 2-methylpropene.
2. Results and discussion
The cross metathesis transformation of methyl oleate (1) with 2-methyl-2-butene (2, Scheme 1) was chosen to obtain potentially valuable commodity chemicals containing prenyl-type double bonds: methyl 10-methylundec-9-enoate (3) and 2-methylundec-2-ene (4).44,48–51 In addition to these expected CM products, the linear by-products of the CM reaction with 2 can also be formed,38 namely, methyl undec-9-enoate (5) and undec-2-ene (6). To make the picture complete, products formed via self-cross metathesis of methyl oleate (1), octadec-9-ene (7) and dimethyl octadec-9-enedioate (8) can also be expected (Scheme 1). The undesired products 5 and 6, created in some amount, can lead in a series of secondary metathesis and isomerisation52,53 events to form even more complicated reaction mixtures. At the same time, because 2-methyl-2-butene (2) is used in excess, we hoped that the less sterically hindered and therefore more metathetically accessible compounds 5 and 6 as well as 7 and 8 can react further with another molecule of 2, thus increasing the amount of the expected products 3 and 4 in the reaction mixture (Scheme 1). Finally, we assumed that the proportion of the possible products obtained from either the alkyl-terminus or the ester-terminus of the methyl oleate molecule considered in pairs can be different (i.e.3vs.4 and 5vs.6), as the consecutive metathesis events are independent.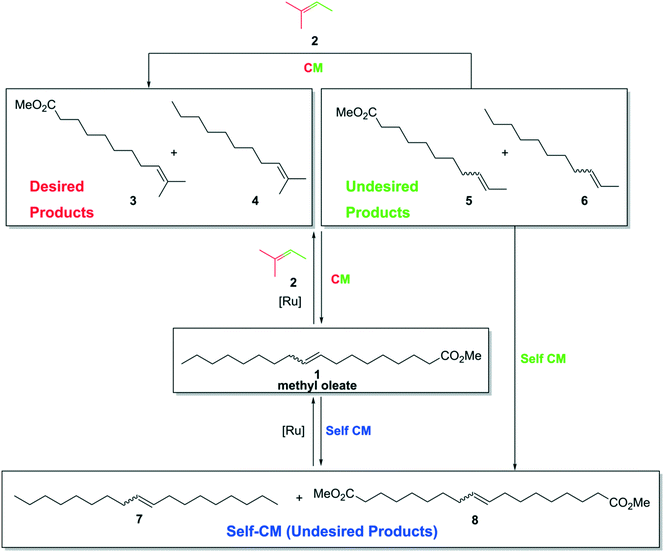 | ||
| Scheme 1 Self-metathesis reaction of methyl oleate (1) and cross-metathesis reaction of methyl oleate (1) with 2-methyl-2-butene (2). | ||
Because the “prenylated” 3 and 4 are the most interesting products from those possible to be found in the reaction mixture,39–45 we assumed that not only the conversion of 1 but also the selectivity towards these two compounds shall be the key factor in our optimisation. To do so, having in hand a large collection of assorted ruthenium metathesis catalysts, we attempted a number of experiments varying the reaction conditions and the catalyst used (Fig. 3 and 11).
2.1 Preliminary study
The first objective of this study was to find the most suitable conditions for the metathesis reaction leading to the highest conversion of 1 and the highest selectivity towards the products 3 and 4. The selection of the best reaction parameters, namely, a solvent, temperature and time, as well as an excess of 2-methyl-2-butene (2), was performed using two very popular and commercially available catalysts: Hoveyda–Grubbs second generation (Hov-II) and Umicore™ M2 (Ind-II) (Fig. 1). The solvents tested were toluene, dichloromethane and dichloroethane; the reaction was also conducted without any solvent. Despite a great interest in carrying out metathesis in aqueous media or in water-based emulsions,54,55 such systems have not been included in the present study. A wide range of temperatures was examined, starting at 20 °C and increasing up to 80 °C with 20° intervals.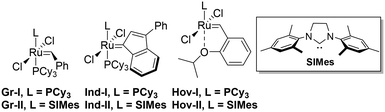 | ||
| Fig. 1 The most popular commercially available ruthenium olefin metathesis catalysts, belonging to the first (L = PCy3) and the second generation (L = SIMes). | ||
During the selection of the most desirable conditions for the metathesis reaction two parameters were considered to be of key importance: the substrate conversion and the molar composition of the resulting reaction mixture. The outcome of the optimization study is presented in Table 1. The conversion of methyl oleate (1) and the proportion of the products in the reaction mixture (presented in mole fractions) were determined by gas chromatography with dodecane as an internal standard, wherein both geometrical isomers E and Z were counted together. To identify the components of the post reaction mixture, the samples were examined by GC-MS (using the same column), and retention times were compared with the independently obtained samples.56 The structure and configuration of the isolated main products were additionally confirmed by 1H and 13C NMR spectroscopy.
| Entry | Cat. | Solvent | Temp [°C] | Time [h] | Equiv. of 2 | Conversion% | Molar fraction of 3b | Molar fraction of 4b | Molar fraction of 5b | Molar fraction of 6b |
|---|---|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: 1 mol% cat., [M](1) = 0.17 M, 0.6 equiv. dodecane (internal standard for GC). b Determined by GC, calculated for both E/Z isomers. c 2 mol% 2-chloroquinone was added. d 2-Methyl-2-butene (2) served as a solvent. | ||||||||||
| 1 | Hov-II | Toluene | Rt | 5 | 2 | 64 | 0.19 | 0.25 | 0.15 | 0.16 |
| 2 | Hov-II | Toluene | Rt | 5 | 10 | 75 | 0.22 | 0.31 | 0.15 | 0.11 |
| 3 | Hov-II | Toluene | Rt | 5 | 19 | 69 | 0.22 | 0.28 | 0.18 | 0.13 |
| 4 | Hov-II | Toluene | Rt | 5 | 38 | 67 | 0.23 | 0.32 | 0.17 | 0.15 |
| 5 | Hov-II | DCM | Rt | 5 | 10 | 40 | 0.19 | 0.29 | 0.17 | 0.08 |
| 6 | Hov-II | DCE | Rt | 5 | 10 | 41 | 0.19 | 0.30 | 0.17 | 0.05 |
| 7 | Hov-II | —d | Rt | 5 | 38 | 77 | 0.24 | 0.32 | 0.17 | 0.12 |
| 8c | Hov II | Toluene | Rt | 5 | 10 | 52 | 0.37 | 0.36 | 0.06 | 0.09 |
| 9c | Hov-II | Toluene | Rt | 24 | 10 | 79 | 0.28 | 0.39 | 0.14 | 0.08 |
| 10 | Hov-II | Toluene | 40 | 5 | 10 | 94 | 0.33 | 0.35 | 0.10 | 0.11 |
| 11 | Hov-II | Toluene | 60 | 5 | 10 | 92 | 0.38 | 0.28 | 0.06 | 0.18 |
| 12 | Hov-II | Toluene | 80 | 5 | 10 | 97 | 0.36 | 0.39 | 0.08 | 0.16 |
| 13 | Ind-II | Toluene | 80 | 5 | 10 | 5 | 0.12 | 0.25 | 0.30 | 0.12 |
| 14 | Ind-II | Toluene | 40 | 5 | 10 | 0 | — | — | — | — |
| 15 | Ind-II | Toluene | 40 | 24 | 10 | 65 | 0.28 | 0.40 | 0.13 | 0.06 |
| 16 | Ind-II | Toluene | 40 | 48 | 10 | 86 | 0.30 | 0.43 | 0.14 | 0.07 |
An initial study on the cross-metathesis of methyl oleate (1) in the presence of the Hoveyda–Grubbs catalyst Hov-II was performed in toluene at room temperature with varying amounts of the cross-partner 2. During these experiments, in order to maintain the concentration of 1 at a constant level, the amount of solvent was decreased to match the increasing amount of 2 (Table 1, entries 1–4). It was found that under these conditions Hov-II exhibited in general similar effectiveness regardless of the quantity of 2-methyl-2-butene (2); however, slightly better conversion and selectivity were observed when the substrate ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. When either dichloromethane or 1,2-dichloroethane was used in the CM reaction instead of toluene under otherwise identical conditions, a significant drop of conversion was observed while selectivity stayed at a similar level (Table 1, entries 5–6). The result of the reaction in which the substrate 2 was also the solvent (Table 1, entry 7) was almost identical to those obtained for the substrate ratio of 1
10. When either dichloromethane or 1,2-dichloroethane was used in the CM reaction instead of toluene under otherwise identical conditions, a significant drop of conversion was observed while selectivity stayed at a similar level (Table 1, entries 5–6). The result of the reaction in which the substrate 2 was also the solvent (Table 1, entry 7) was almost identical to those obtained for the substrate ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (Table 1, entry 2), but for economic reasons such conditions were not considered further. Since it was proved52 that addition of quinones may improve the selectivity of the metathesis reaction, 2-chlorobenzoquinone was examined (Table 1, entries 8 and 9). The first reaction was performed in toluene at room temperature for 5 h with ten equivalents of 2 relative to 1. An increase in selectivity of the process towards the products 3 and 4 was observed; however, the conversion was more than 20% lower than that observed in the reaction without quinone (Table 1, entry 2). When the reaction time was extended to 24 h the conversion increased, but at the same time the selectivity decreased. Because in our opinion the benefits achieved by the introduction of an additional reagent to the examined system did not compensate for its additional complication, studies utilizing quinones were not continued. Next, the influence of temperature was examined (Table 1, entries 2, 10–12). It was found that increasing the temperature from rt to 40 °C resulted in an improvement both in conversion and in selectivity. A further increase of the temperature to 60 and 80 °C resulted in almost full conversion and good selectivity.
10 (Table 1, entry 2), but for economic reasons such conditions were not considered further. Since it was proved52 that addition of quinones may improve the selectivity of the metathesis reaction, 2-chlorobenzoquinone was examined (Table 1, entries 8 and 9). The first reaction was performed in toluene at room temperature for 5 h with ten equivalents of 2 relative to 1. An increase in selectivity of the process towards the products 3 and 4 was observed; however, the conversion was more than 20% lower than that observed in the reaction without quinone (Table 1, entry 2). When the reaction time was extended to 24 h the conversion increased, but at the same time the selectivity decreased. Because in our opinion the benefits achieved by the introduction of an additional reagent to the examined system did not compensate for its additional complication, studies utilizing quinones were not continued. Next, the influence of temperature was examined (Table 1, entries 2, 10–12). It was found that increasing the temperature from rt to 40 °C resulted in an improvement both in conversion and in selectivity. A further increase of the temperature to 60 and 80 °C resulted in almost full conversion and good selectivity.
Interestingly, when the indenylidene catalyst Umicore™ M2 (Ind-II) was applied under the conditions optimized for the Hoveyda–Grubbs complex (10 equivalents of 2, toluene, 80 °C, 5 h), almost no conversion of substrate was observed (Table 1, entry 14). This forced us to re-investigate the optimal conditions for the indenylidene system57 in an independent way. Since toluene appeared to be the best solvent for the CM reaction of 1, and the ratio between substrates 1 and 2 seemed to be optimal as well, only the temperature, the reaction time and the amount of catalyst were re-examined during this optimization. As was found previously, when the reaction was performed for 5 hours at 80 °C, only 5% conversion was observed and, as expected, decreasing the temperature to 40 °C gave an even worse result—practically no conversion. However, when the reaction time was extended to 24 and 48 h the level of conversion increased significantly to 65% and 86% (Table 1, entries 15 and 16). Based on these results the optimal conditions for further research were selected, namely, 10 equivalents of 2, toluene as a solvent, 5 hours at 80 °C for Hoveyda–Grubbs type complexes and 48 hours at 40 °C for indenylidene. The latter was also applied for Grubbs type catalysts since it is known that most complexes of this type exhibit limited stability at elevated temperature.
Interestingly, migration of the double bond was not observed in the examined reactions.
2.2 First generation complexes
With the optimized reaction conditions in hand, the systematic search for the best catalyst for the cross-metathesis reaction between methyl oleate (1) and 2-methyl-2-butene (2) was started. First, the reaction catalysed by first generation complexes (Fig. 2) was studied.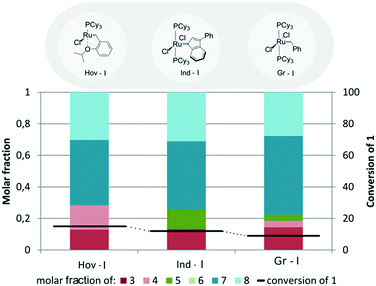 | ||
| Fig. 2 Comparison of 1st generation catalysts (conditions: 2 – 10 equiv., [Ru] – 1 mol%, toluene, 5 h, 80 °C for Hov I and 48 h at 40 °C for Gr I and Ind I). | ||
In comparison to previously utilized second generation complexes, the first generation catalysts Hov-I, Ind-I, and Gr-I were found to be selective mostly towards the undesired self-metathesis reaction, as the “dimerization” products 7 and 8 comprised around 80% of all products present in the reaction mixture. What is more, first generation complexes were much less active than the previously examined second generations catalysts (see Table 1, entry 12 for Hov-II and entry 16 for Ind-II) and did not even reach 20% conversion (15, 12 and 9% for Hov-I, Ind-I, and Gr-I, respectively).
2.3 Phosphine-free second generation complexes
Since the first generation complexes were found to be useless catalysts in the studied transformation, we decided to concentrate our efforts on finding the optimal Ru-compound promoting the CM reaction among second-generation catalysts, both the commercially available ones and those recently obtained in our laboratory. The goal behind testing a so large and heterogeneous collection of catalysts was to find the correlation between the catalyst structure and their activity and selectivity in the studied CM reaction between 1 and 2. Particular emphasis was put on the impact of different NHC and benzylidene ligands present in ruthenium complexes (Fig. 3).In a series of cross-metathesis reactions of methyl oleate (1) with 2-methyl-2-butene (2) utilizing Hoveyda-Grubbs type complexes mixed results were observed (Fig. 4). There were catalysts that exhibited very high activity, namely, Hov-II, [Ru]-4, [Ru]-6, [Ru]-7, [Ru]-12, [Ru]-14, [Ru]-15, [Ru]-16, [Ru]-21, and [Ru]-23 (Fig. 4), but at the same time complexes that converted only a small fraction of 1 into products were found: [Ru]-2, [Ru]-8, and [Ru]-11. These experiments provided the first input for a detailed study on the influence of the structure of both neutral and benzylidene-type ligands on the catalyst activity and selectivity.
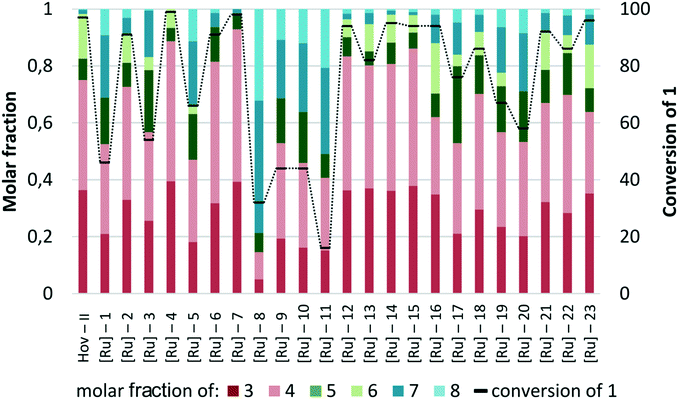 | ||
| Fig. 4 Comparison of various Hoveyda–Grubbs type catalysts (conditions: 2 – 10 equiv., [Ru] – 1 mol%, toluene, 5 h, 80°). | ||
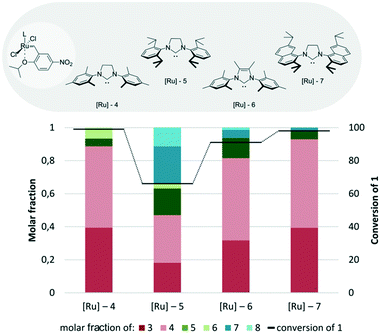
First, the influence of different NHC ligands on the activity of Hoveyda–Grubbs type catalysts bearing the same benzylidene part was examined (Fig. 5). Compared to the commercially available Hoveyda–Grubbs catalyst Hov-II bearing the SIMes ligand (1,3-bis(2,4,6-trimethylphenyl)imidazolin-2-ylidene), its analogue with SIPr (1,3-bis(2,6-diisopropylphenyl)imidazolin-2-ylidene), [Ru]-1, exhibited lower activity and selectivity, as the undesired products 5, 7, and 8 accounted for around 50% of all products. A rather similar result was obtained for [Ru]-3 bearing a methyl 2,3,4,5,6-pentafluorobenzoate substituent in the backbone of 4,5-dihydro-1H-imidazole, which was a small disenchantment.58 Exchanging SIMes with Me2IMes (1,3-bis(2,4,6-trimethylphenyl)imidazol-2-ylidene) ([Ru]-2)59 also led to a significant decrease in both conversion and selectivity of the cross-metathesis reaction.
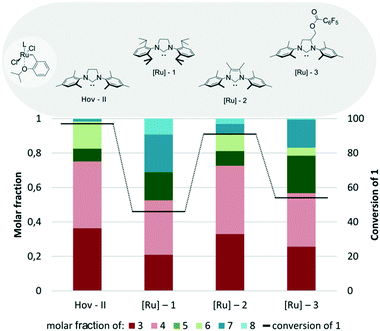 | ||
| Fig. 5 Comparison of Hoveyda–Grubbs type catalysts bearing different NHC ligands (conditions as in Fig. 4). | ||
The next vital structural feature checked by us was the influence of an alkoxy fragment in the benzylidene ligand of the Hoveyda–Grubbs catalyst. To do so, complexes [Ru]-10, [Ru]-16, [Ru]-17, [Ru]-18, and [Ru]-19 were compared in the CM of 1 with 2 with Hov-II (Fig. 6). Unfortunately, replacing the isopropoxy fragment of the original Hoveyda catalyst with other alkoxy groups led to catalysts of decreased activity and selectivity (Fig. 6). In almost each case significant amounts of the homo-dimerization products 7 and 8 or undesired cross-metathesis products 5 and 6 were formed. In terms of catalytic activity the most advantageous change was the replacement of isopropyl by the cyclopentane group in the ethereal fragment, as demonstrated by complex [Ru]-16.60,61 Application of this catalyst resulted in 96% conversion of 1; however the selectivity of the studied CM process was lower than in the case of iPrO-substituted Hov-II. Interestingly, use of this catalyst led to larger quantities of cross-metathesis products 5 and 6 bearing disubstituted double bonds. When a higher “homologue” of [Ru]-16, namely [Ru]-17, was utilized, both conversion and selectivity slightly dropped, although they still remained at an acceptable level. Finally, in the model reaction between 1 and 2 catalysed by the commercially available Umicore™ M51 ([Ru]-18),62 the observed conversion was comparable to the result obtained with [Ru]-16 (86 versus 94%) while selectivity towards the desired CM products was slightly higher. On the other hand, Umicore™ M52 ([Ru]-19),62 which differs from M51 only by the lack of a methyl group, was less effective and exhibited both lower conversion and selectivity. The least effective complex in this series was [Ru]-10,63 which at about 45% conversion gave an almost equimolar mixture of 3–5, 7 and 8.
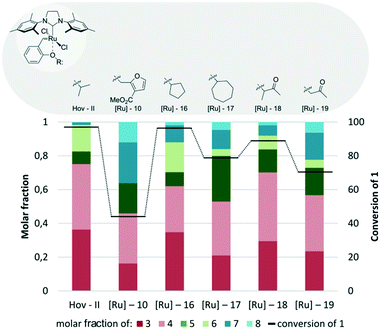 | ||
| Fig. 6 Comparison of Hoveyda–Grubbs type catalysts with different ethereal substituents in the benzylidene moiety (conditions as in Fig. 4). | ||
Until now the SIMes bearing Hov-II has been the most active and selective among the tested catalysts, transforming 1 very effectively to the desired products 3 and 4. In the next step, the influence of electronic modification64 of the benzylidene ligand by introducing electron withdrawing groups (EWGs) in the para position to the isopropoxy group was examined (Fig. 7). It is known that EWGs could improve the catalytic activity of the Hoveyda–Grubbs complex due to the weakening of the oxygen–ruthenium bond and facilitation of dissociation of benzylidene ligands.64 The first member of this family of EWG-activated metathesis catalysts, [Ru]-4 with a nitro substituent,65 exhibited indeed a visibly higher activity than that observed for Hov-II (Fig. 7). Furthermore, only a very small amount of side products was formed. Slightly lower but still fairly satisfactory activity and selectivity were exhibited by the commercially available Umicore™ M71 SIMes ([Ru]-12) with the 2,2,2-trifluoro-N-methylacetamide substituent as well as by Umicore™ M73 SIMes ([Ru]-14) with the carbamate substituent.62 When M73 SIMes was utilized in the studied reaction, good conversion of 1 (92%) as well as good selectivity was observed; in the reaction mixture mainly cross-metathesis products were present with a small amount of dimeric products. An even better result was found for the structural analogue [Ru]-12 (Umicore™ M71 SIMes); the selectivity slightly increased and the conversion of 1 was higher by a few percent. The Zannan catalyst [Ru]-23,66 with a dimethylsulfonamide substituent, was one of the most active among the tested complexes, giving 96% conversion; however in terms of selectivity it exhibited worse properties than those of its competitors, as a significant amount of dimerization products were formed during the reaction (Fig. 7). The last members of the group of EWG substituted Hoveyda–Grubbs analogues were complexes bearing benzenesulfonic esters, [Ru]-20, [Ru]-21 and [Ru]-22 (in Fig. 7 only the best one is shown).67 In accordance with the literature notion, we observed that the substituent in the para position of benzenesulfonic acid has a significant influence on the catalyst activity (Fig. 7). Complex [Ru]-20 was found to be the least active and selective one in this group, while both [Ru]-21 and [Ru]-22, where the phenyl sulfone fragment was modified either with a methoxy or a nitro group, show substantially better results in terms of conversion (92 and 86%, respectively, in comparison to 58% for [Ru]-20) and selectivity.
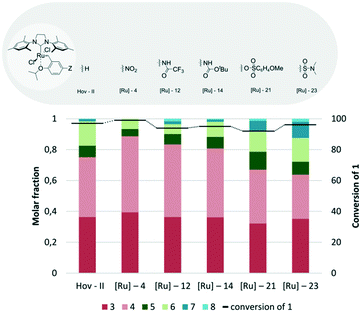 | ||
| Fig. 7 Comparison of Hoveyda–Grubbs analogues with SIMes ligand bearing different benzylidene moieties (conditions as in Fig. 4). | ||
As the nitro-substituted Hoveyda catalyst [Ru]-4 was found to exhibit the highest conversion and selectivity in the studied CM of 1, we decided to use it as the platform to investigate the influence of various NHC ligands in more detail (Fig. 8). The first alteration of the nitro-Hoveyda catalyst was complex [Ru]-5 with a sterically enlarged NHC ligand (SIPr). This modification, however, exhibited rather moderate activity, with 60% substrate conversion, and moderate selectivity. On the other hand, a designer imidazolinium NHC ligand, introduced by Dorta68,69 (as in complex [Ru]-7) led to more satisfactory results. Application of [Ru]-7 gave an almost quantitative conversion together with high selectivity towards the desired products 3 and 4. The dimethylimidazolium NHC ligand,59 placed on the same platform ([Ru]-6) gave still satisfactory results, but its performance was visibly lesser.
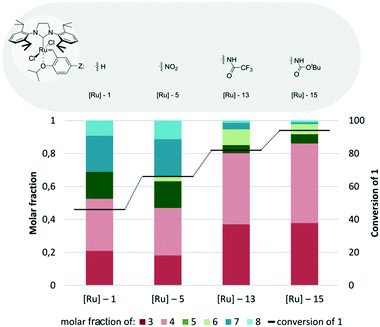 | ||
| Fig. 8 Comparison of [Ru]-4 analogues bearing different NHC ligands (conditions as in Fig. 4). | ||
As could be expected from the preliminary screening (cf.Fig. 5) most of the substituted Hoveyda–Grubbs type catalysts bearing the SIPr ligand showed average activity in CM of methyl oleate (1) and 2-methyl-2-butene (2) (Fig. 9). Only commercially available [Ru]-13 (Umicore™ M71-SIPr) and [Ru]-15 (Umicore™ M73-SIPr)62 led to satisfactory results. However, the activity and selectivity exhibited by these complexes were still lower than those of SIMes containing [Ru]-4.
 | ||
| Fig. 9 Comparison of Hoveyda–Grubbs type catalysts with different benzylidene moieties and SIPr NHCs (conditions as in Fig. 4). | ||
In the next step of our study, the influence of anionic ligands on the model CM reaction was tested (Fig. 10). When chloride ligands in Hov-II were changed to trifluoroacetic ones, producing the modified Hoveyda–Grubbs type catalyst [Ru]-8,70 a significant reduction of the usefulness of such an obtained complex in the cross-metathesis reaction between methyl oleate (1) and 2-methyl-2-butene (2) was observed. Not only did the conversion decrease by almost two thirds, but also a significant decrease of the selectivity towards the desired compounds 3 and 4 was detected, while 7 and 8 were the main components of the reaction mixture. In contrast to complexes bearing the SIMes ligand, in the case of their SIPr analogues, the modification of the anionic ligands affected neither the activity nor the selectivity, as the results obtained for [Ru]-1 and [Ru]-9 were almost the same.
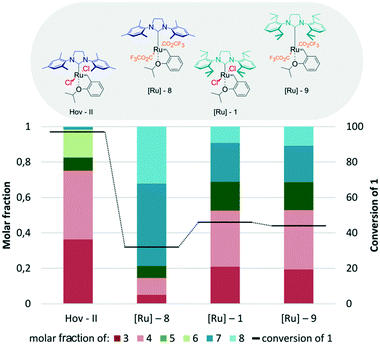 | ||
| Fig. 10 Hoveyda–Grubbs type catalysts bearing different anionic ligands (conditions as in Fig. 4). | ||
2.4 Second generation complexes with phosphine ligand
In the second part of the study, we attempted to test in more detail complexes other than phosphine-free Hoveyda–Grubbs catalysts (Fig. 11). Since in the preliminary study we noted that the cross-metathesis reaction of methyl oleate (1) and 2-methyl-2-butene (2) in the presence of indenylidene catalyst Ind-II was significantly slower as compared to the same transformation catalysed by Hoveyda–Grubbs complex, in this part of the research the reaction time was extended to 48 hours and the temperature was maintained at 40 °C.After some preliminary experiments, we decided to apply the same conditions also to reactions promoted by Grubbs type catalysts. The results of testing of the small library of 18 Grubbs and indenylidene type of complexes require some comments. There were not only catalysts with relatively high activity, which resulted in a high level of conversion of methyl oleate (1), viz.Ind-II, [Ru]-27, [Ru]-32, [Ru]-33, [Ru]-37, and [Ru]-38, but also catalysts characterized by very low activity with the conversion of 1 below 20%, viz.[Ru]-25, [Ru]-26, [Ru]-30, [Ru]-34, and [Ru]-35 (Fig. 12). Disappointingly, most of the indenylidene and Grubbs type complexes exhibited low selectivity and in most cases the percentage of the desired products in the reaction mixture was less than 50%. Only two complexes produced a high yield of the products 3 and 4, namely Ind-II and [Ru]-37.
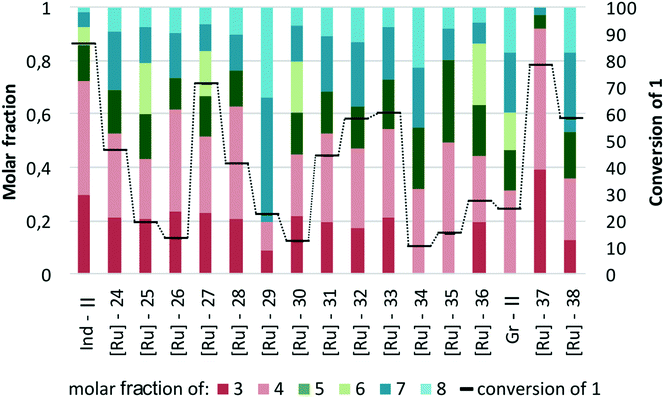 | ||
| Fig. 12 Comparison of the indenylidene and Grubbs type catalysts (conditions: 2 – 10 equiv., [Ru] – 1 mol%, toluene, 48 h at 40 °C). | ||
Analysis of the reaction mixtures presented in Fig. 13 clearly shows that commercial Umicore™ M2 catalyst Ind-II is the most active complex in the tested transformation, while its structural modification caused a decrease in the conversion of substrate 1. Importantly, Ind-II was also one of the most selective in its class (Fig. 13). When Ind-II bearing the saturated SIMes ligand was substituted with IMes containing [Ru]-25, a significant decrease in activity of the latter was observed, resulting in a much lower conversion and yield of the desired products. Application of Me2IMes-containing [Ru]-2459 allows for a twofold increase in conversion as compared with [Ru]-25; however, it was still only half of the number obtained for Ind-II. Both ruthenium complexes bearing unsaturated ligands reached very similar selectivity and gave a mixture of cross- and self-metathesis products. Introduction of a substituent into the backbone of 4,5-dihydro-1H-imidazole ligand, as in [Ru]-33,71 resulted in higher conversion of 1 and higher selectivity towards 3 and 4, but still gave worse results as compared to unmodified Ind-II. Complex [Ru]-2672 was the least active one in this series; however, it was also among the most selective ones.
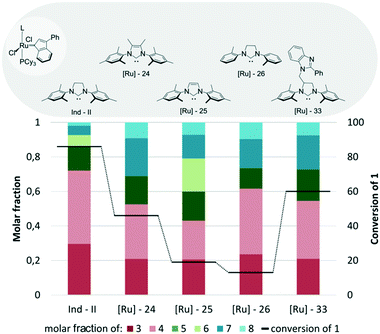 | ||
| Fig. 13 Comparison of Ind-II analogues bearing different NHC ligands (both N-substituents in the imidazole/imidazoline ring are aromatic) (conditions as in Fig. 12). | ||
In Fig. 14 we present in greater detail a subset of indenylidene catalysts bearing unsymmetrical NHC ligands. It is easy to see that modification of the N-substituent in the imidazolinium ring of a NHC ligand has a huge influence on the effectivity of such derived catalysts in cross-metathesis reactions between 1 and 2 (Fig. 14). For example, replacement of one mesitylene ring with a benzyl group possessing a substituent in the ortho position, such as the N,N-dimethylamino group in [Ru]-2773 or the methoxy group in [Ru]-28,73 reduced the conversion of the methyl oleate (1) to 71 and 41%, respectively, in comparison to 86% obtained when unmodified Umicore M2 was applied. The same trend was even more visible for the complexes containing thiophene or furane moieties, [Ru]-2973 and [Ru]-30,73 respectively, where the conversion was as little as 22 and 12%. The changes in the structure of the NHC ligand also affected the selectivity of the process. When complexes with N-benzyl NHC ligands were applied, a mixture of cross- and self-metathesis products was obtained; however, in both cases 3 and 4 were formed predominantly. Interestingly, application of catalyst [Ru]-29 bearing the NHC ligand with a furane moiety gave in large proportion products of self-CM of 1 (however with low conversion). Having in hand two mass-enlarged complexes designed for application in nanofiltration,74,75 we decided to include them in our tests. Unfortunately, when [Ru]-31 and [Ru]-32 bearing NHC ligands decorated with polyhedral oligomeric silsesquioxane (POSS) were applied, the conversion of substrate 1 reached 40–60% only, and the selectivity was rather low.
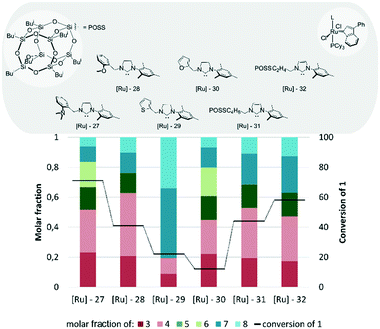 | ||
| Fig. 14 Comparison of Umicore™ M2 analogues bearing different NHC ligands (one arm of the imidazole/imidazoline ring is aliphatic) (conditions as in Fig. 12). | ||
The next factor which strongly affected the results of cross-metathesis reactions between methyl oleate (1) and 2-methyl-2-butene (2) catalysed by indenylidene type complexes was the kind of P-ligand used (Fig. 15). The highest conversion (86%) and selectivity towards the desired products 3 and 4 were exhibited by Ind-II, the catalyst bearing a tricyclohexylphosphine ligand. Exchange of tricyclohexylphosphine with triphenylphosphine (in [Ru]-35) or triisopropylphosphite (in [Ru]-36) caused a significant drop in conversion to 15% and 27%, respectively. In contrast to Ind-II which gave predominantly a mixture of both cross-metathesis products, [Ru]-35 was selective towards only one of them, namely 2-methylundec-2-ene (4). This product represents approximately 50% of all products obtained during the reaction. [Ru]-36 provided an almost equimolar mixture of “prenylated” (3 and 4) and linear (5 and 6) products of cross-metathesis reaction together with around 10% self-products, 7 and 8. The somewhat disappointing result obtained with [Ru]-36, which previously was proved to be an effective catalyst for challenging reactions operating at high temperatures (80° or higher),76 is due to the utilization of this complex below the optimal temperature for its activity. Although we are convinced that an increase in temperature to 80 or even 120 °C would considerably improve the result, for the sake of an easier comparison with other complexes we kept the conditions fixed. It should also be mentioned that the studied complex [Ru]-36 is in the cis form while the other coupled catalysts are in the trans form. [Ru]-35, in which the only structural modification in comparison to [Ru]-34 was the replacement of the SIMes ligand by the SIPr one, exhibited slightly lower activity and selectivity than its analogue.
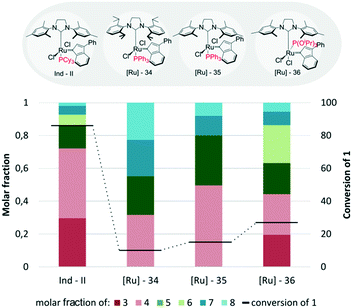 | ||
| Fig. 15 Indenylidene type catalysts bearing other neutral ligands (conditions as in Fig. 12). | ||
The Grubbs type complexes (Fig. 16) were found to be relatively ineffective in the reaction of methyl oleate (1) and 2-methyl-2-butene (2) in comparison to Hoveyda–Grubbs catalysts even if the reaction time was almost ten times longer. The first generation catalyst Gr-I, as shown previously in Fig. 2, exhibited low activity and formed predominantly self-metathesis products. In comparison, the second generation complexes were more reactive and transformed 1 with moderate efficiency; however they favoured the formation of the self-metathesis products 7 and 8 at the expense of the desired products. The only exception was [Ru]-37 bearing a Me2IMes ligand which not only was the most active (conversion 78%) but also was the most selective catalyst in the tested group. Mainly the cross-metathesis products 3 and 4 were obtained with only traces of dimerization products in this case; however, the conversion was lower than in the case of the Hoveyda–Grubbs system.
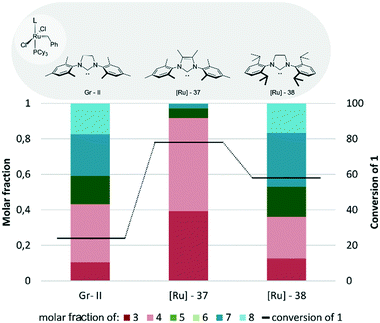 | ||
| Fig. 16 Grubbs type complexes (conditions as in Fig. 12). | ||
After a thorough and meticulous testing of numerous catalysts the best ruthenium complex for cross-metathesis reaction of methyl oleate (1) with 2-methyl-2-butene (2) was selected—[Ru]-4. Not only did it give very good results—almost quantitative conversion and very good selectivity—but it is also commercially available (comparable results were obtained for [Ru]-7, but since it was a “homemade” catalyst it has limited availability). That is why [Ru]-4 was selected for further study with decreased catalyst loading (0.5 and 0.1 mol% and then 500 and 100 ppm, Fig. 17). When 0.5 mol% [Ru]-4 was used, the conversion of the starting material was very similar to the result obtained in the presence of 1 mol% catalysts. Also, decreasing the catalysts loading to 0.1% resulted in relatively good conversion of 1 (81%) but further reducing the amount of complex failed—no products of the metathesis reaction were observed. Another interesting observation was a drop in selectivity caused by diminishing catalyst loading. This can be related to the fact that during the metathesis process 1 and 2 reacted with each other, yielding both main and side products (see Scheme 1). The main products 3 and 4, having triple-substituted double bonds, did not undergo further reaction but at the same time by-products 5–8 with double-substituted double bonds could still react. The conditions selected for the examined transformation with excess 2-methyl-2-butene (2) and a sufficient amount of the catalyst allowed for further reaction of the side-products that were created at the beginning of the reaction with an excess of 2 while the metathesis catalyst was still active. When a smaller loading of the complex was used, not all products had “enough time” to react, with an excess of 2 leading to major CM products.
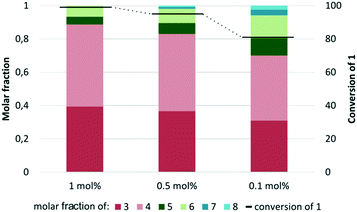 | ||
| Fig. 17 Cross metathesis reaction with different loadings of [Ru]-4 (conditions: 2 – 10 equiv., toluene, 5 h, 80 °C). At 500 and 100 ppm loading no conversion was observed. | ||
Conclusions
Screening a large set of various Ru metathesis catalysts in a cross-metathesis reaction of methyl oleate (1) with 2-methyl-2-butene (2) allowed for selecting complexes that exhibited the most advantageous properties in this transformation, leading to high selectivity toward the desired cross-metathesis products 3 and 4 bearing a trisubstituted double bond. Although no simple relationships between the structure of the complexes and their effectiveness in CM of 1 was found, some trends however were noticed. First of all, it was found that the CM reaction could be effectively performed only with second generation Ru catalysts. When the first generation complexes were applied, a significant decrease in activity was observed together with an increase in selectivity towards unwanted self-metathesis products. In comparison to indenylidene and Grubbs systems, Hoveyda–Grubbs catalysts were found to be, in general, more active, reaching higher conversion in a shorter time with only just a few exceptions. When the reaction was carried out with less active complexes, the self-metathesis products 7 and 8 were predominantly formed, which is particularly true for the majority of Grubbs and indenylidene type catalysts. Under standard conditions (2 – 10 equiv., [Ru] – 1 mol%, toluene, 5 h, 80°) the catalyst of choice for this transformation is [Ru]-4 which not only gave one of the best results (together with [Ru]-7) but is also commercially available. Decreasing the catalyst loading to 1000 ppm allowed for synthesis of the desired products with a high yield and acceptable selectivity. Further reduction of the amount of catalysts failed, and only substrates were observed in the reaction mixture.The obtained results clearly demonstrate that one can effectively synthesize olefins with a prenylated double bond; however, the transformation is challenging because the reaction mixture obtained is a complex one. On the other hand, in the present study the suitable conditions and a high-performing catalyst for this reaction were identified.
Acknowledgements
AK would like to thank The National Science Centre – Poland, Program SONATA, NCN/2011/03/D/ST5/06079, for financial support. KG and AS wish to thank the Foundation for Polish Science for the “Team” Programme co-financed by the EU European Regional Development Fund-Operational Program Innovative Economy 2009/2013.References
- K. Grela, Olefin Metathesis: Theory and Practice, John Wiley & Sons, Inc., Hoboken, 2014 Search PubMed.
- R. H. Grubbs, A. G. Wenzel, D. J. O'Leary and E. Khosravi, Handbook of Metathesis, Wiley-VCH, Weinheim, 2014 Search PubMed.
- H. M. Li, J. P. Scott, C. Y. Chen, M. Journet, K. Belyk, J. Balsells, B. Kosjek, C. A. Baxter, G. W. Stewart, C. Wise, M. Alam, Z. J. Song and L. S. Tan, Org. Lett., 2015, 17, 1533–1536 CrossRef PubMed.
- Y. Jiang, S. W. Andrews, K. R. Condroski, B. Buckman, V. Serebryany, S. Wenglowsky, A. L. Kennedy, M. R. Madduru, B. Wang, M. Lyon, G. A. Doherty, B. T. Woodard, C. Lemieux, M. G. Do, H. Zhang, J. Ballard, G. Vigers, B. J. Brandhuber, P. Stengel, J. A. Josey, L. Beigelman, L. Blatt and S. D. Seiwert, J. Med. Chem., 2014, 57, 1753–1769 CrossRef CAS PubMed.
- O. Nuyken and S. Pask, Polymers, 2013, 5, 361 CrossRef.
- A. Leitgeb, A. Szadkowska, M. Michalak, M. Barbasiewicz, K. Grela and C. Slugovc, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 3448–3454 CrossRef CAS.
- A. A. Nagarkar and A. F. M. Kilbinger, Nat. Chem., 2015, 7, 718–723 CrossRef CAS PubMed.
- H. J. Meyer, Dalton Trans., 2010, 39, 5973–5982 RSC.
- N. J. Takas, L. E. Slomka, X. Yang, N. Giles and J. A. Aitken, J. Solid State Chem., 2008, 181, 3044–3050 CrossRef CAS.
- N. Cerit, N. Cakir, A. Dag, O. Sirkecioglu, H. Durmaz, G. Hizal and U. Tunca, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 2850–2858 CrossRef CAS.
- H. Mutlu, L. M. de Espinosa and M. A. R. Meier, Chem. Soc. Rev., 2011, 40, 1404–1445 RSC.
- V. P. Kamat, H. Hagiwara, T. Katsumi, T. Hoshi, T. Suzuki and M. Ando, Tetrahedron, 2000, 56, 4397–4403 CrossRef CAS.
- X. Yu and D. Sun, Molecules, 2013, 18, 6230 CrossRef CAS PubMed.
- A. Michrowska, M. Bieniek, M. Kim, R. Klajn and K. Grela, Tetrahedron, 2003, 59, 4525–4531 CrossRef CAS.
- X. Miao, A. Blokhin, A. Pasynskii, S. Nefedov, S. N. Osipov, T. Roisnel, C. Bruneau and P. H. Dixneuf, Organometallics, 2010, 29, 5257–5262 CrossRef CAS.
- C. Bruneau, C. Fischmeister, X. Miao, R. Malacea and P. H. Dixneuf, Eur. J. Lipid Sci. Technol., 2010, 112, 3–9 CrossRef CAS.
- M. A. R. Meier, Lipid Technol., 2008, 20, 84–87 CrossRef CAS.
- M. A. R. Meier, Macromol. Chem. Phys., 2009, 210, 1073–1079 CrossRef CAS.
- V. Sashuk, C. Samojlowicz, A. Szadkowska and K. Grela, Chem. Commun., 2008, 2468–2470 RSC.
- T. T. Nguyen, M. J. Koh, X. Shen, F. Romiti, R. R. Schrock and A. H. Hoveyda, Science, 2016, 352, 569–575 CrossRef CAS PubMed.
- Report of the United Nations Conference on Enviormental Development, Rio de Janeiro (Brazil) http://www.un.org/esa/sustdev, Accessed 1992.
- H. Villar, M. Frings and C. Bolm, Chem. Soc. Rev., 2007, 36, 55–66 RSC.
- R. Malacea and P. H. Dixneuf, in Green Metathesis Chemistry, ed. V. Dragutan, A. Demonceau, I. Dragutan and E. S. Finkelshtein, Springer Netherlands, Dordrecht, 2010, pp. 185–206 Search PubMed.
- M. R. Infante, L. Pérez, M. C. Morán, R. Pons, M. Mitjans, M. P. Vinardell, M. T. Garcia and A. Pinazo, Eur. J. Lipid Sci. Technol., 2010, 112, 110–121 CrossRef CAS.
- K. Kosswig, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2000, p. 747 Search PubMed.
- G. D. Yadav and N. S. Doshi, Green Chem., 2002, 4, 528–540 RSC.
- F. N. Jones, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2000, p. 409 Search PubMed.
- J. Schäfer Hans, M. Harenbrock, E. Klocke, M. Plate and A. Weiper-Idelmann, Pure Appl. Chem., 2007, 79, 2047 Search PubMed.
- S. Grinberg, N. Kipnis, C. Linder, V. Kolot and E. Heldman, Eur. J. Lipid Sci. Technol., 2010, 112, 137–151 CrossRef CAS.
- D. J. Cole-Hamilton, Angew. Chem., Int. Ed., 2010, 49, 8564–8566 CrossRef CAS PubMed.
- A. Rybak, P. A. Fokou and M. A. R. Meier, Eur. J. Lipid Sci. Technol., 2008, 110, 797–804 CrossRef CAS.
- A. Nickel, T. Ung, G. Mkrtumyan, J. Uy, C. Lee, D. Stoianova, J. Papazian, W.-H. Wei, A. Mallari, Y. Schrodi and R. Pederson, Top. Catal., 2012, 55, 518–523 CrossRef CAS.
- K. A. Burdett, L. D. Harris, P. Margl, B. R. Maughon, T. Mokhtar-Zadeh, P. C. Saucier and E. P. Wasserman, Organometallics, 2004, 23, 2027–2047 CrossRef CAS.
- J. Patel, S. Mujcinovic, W. R. Jackson, A. J. Robinson, A. K. Serelis and C. Such, Green Chem., 2006, 8, 450–454 RSC.
- A. Behr and J. Pérez Gomes, Beilstein J. Org. Chem., 2011, 7, 1–8 CrossRef CAS PubMed.
- A. Behr and S. Toepell, J. Am. Oil Chem. Soc., 2015, 92, 603–611 CrossRef CAS.
- A. Kajetanowicz, A. Sytniczuk and K. Grela, Green Chem., 2014, 16, 1579–1585 RSC.
- A. K. Chatterjee, D. P. Sanders and R. H. Grubbs, Org. Lett., 2002, 4, 1939–1942 CrossRef CAS PubMed.
- J. J. Ritter and P. P. Minieri, J. Am. Chem. Soc., 1948, 70, 4045–4048 CrossRef CAS PubMed.
- K. Speck, K. Karaghiosoff and T. Magauer, Org. Lett., 2015, 17, 1982–1985 CrossRef CAS PubMed.
- R. J. Comito, F. G. Finelli and D. W. C. MacMillan, J. Am. Chem. Soc., 2013, 135, 9358–9361 CrossRef CAS PubMed.
- C. Obradors, D. Leboeuf, J. Aydin and A. M. Echavarren, Org. Lett., 2013, 15, 1576–1579 CrossRef CAS PubMed.
- C. Ko, J. B. Feltenberger, S. K. Ghosh and R. P. Hsung, Org. Lett., 2008, 10, 1971–1974 CrossRef CAS PubMed.
- K. Mihara, I. Okada, K. Chiba and Y. Kitano, Synthesis, 2014, 46, 1455–1462 CrossRef.
- P. J. Casey and M. C. Seabra, J. Biol. Chem., 1996, 271, 5289–5292 CrossRef CAS PubMed.
- S. Tischer and P. Metz, Adv. Synth. Catal., 2007, 349, 147–151 CrossRef CAS.
- M. Uwamori, A. Saito and M. Nakada, J. Org. Chem., 2012, 77, 5098–5107 CrossRef CAS PubMed.
- D. C. Whitehead, B. R. Travis and B. Borhan, Tetrahedron Lett., 2006, 47, 3797–3800 CrossRef CAS.
- Y.-S. Hon, S.-W. Lin, L. Lu and Y.-J. Chen, Tetrahedron, 1995, 51, 5019–5034 CrossRef CAS.
- S. W. M. Crossley, F. Barabé and R. A. Shenvi, J. Am. Chem. Soc., 2014, 136, 16788–16791 CrossRef CAS PubMed.
- G. Cahiez, C. Duplais and A. Moyeux, Org. Lett., 2007, 9, 3253–3254 CrossRef CAS PubMed.
- S. H. Hong, D. P. Sanders, C. W. Lee and R. H. Grubbs, J. Am. Chem. Soc., 2005, 127, 17160–17161 CrossRef CAS PubMed.
- S. H. Hong, A. G. Wenzel, T. T. Salguero, M. W. Day and R. H. Grubbs, J. Am. Chem. Soc., 2007, 129, 7961–7968 CrossRef CAS PubMed.
- J. Tomasek and J. Schatz, Green Chem., 2013, 15, 2317–2338 RSC.
- D. Burtscher and K. Grela, Angew. Chem., Int. Ed., 2009, 48, 442–454 CrossRef CAS PubMed.
- Y. Kwon, S. Lee, D.-C. Oh and S. Kim, Angew. Chem., Int. Ed., 2011, 50, 8275–8278 CrossRef CAS PubMed.
- F. Boeda, H. Clavier and S. P. Nolan, Chem. Commun., 2008, 2726–2740 RSC.
- S. J. Czarnocki and K. Grela, unpublished work.
- M. Bieniek, K. Grela, A. Rosiak, J. Tarabocchia, A. Szadkowska and R. Kadyrov, in Catalysis of Organic Reactions, CRC Press, 2008, pp. 217–222 Search PubMed.
- K. M. Engle, G. Lu, S.-X. Luo, L. M. Henling, M. K. Takase, P. Liu, K. N. Houk and R. H. Grubbs, J. Am. Chem. Soc., 2015, 137, 5782–5792 CrossRef CAS PubMed.
- K. M. Engle, S.-X. Luo and R. H. Grubbs, J. Org. Chem., 2015, 80, 4213–4220 CrossRef CAS PubMed.
- Umicore Ru-complexes, www.umicore.com.
-
G. Szczepaniak and K. Grela,
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) unpublished work.
unpublished work. - T. K. Olszewski, M. Bieniek, K. Skowerski and K. Grela, Synlett, 2013, 24, 903–919 CrossRef CAS.
- A. Michrowska, R. Bujok, S. Harutyunyan, V. Sashuk, G. Dolgonos and K. Grela, J. Am. Chem. Soc., 2004, 126, 9318–9325 CrossRef CAS PubMed.
- STREM Ru-complexes, www.strem.com.
- M. Smoleń, M. Kędziorek and K. Grela, Catal. Commun., 2014, 44, 80–84 CrossRef.
- L. Vieille-Petit, H. Clavier, A. Linden, S. Blumentritt, S. P. Nolan and R. Dorta, Organometallics, 2010, 29, 775–788 CrossRef CAS.
- D. Gallenkamp and A. Fürstner, J. Am. Chem. Soc., 2011, 133, 9232–9235 CrossRef CAS PubMed.
- L. Yang, M. Mayr, K. Wurst and M. R. Buchmeiser, Chem. – Eur. J., 2004, 10, 5761–5770 CrossRef CAS PubMed.
- M. Kluciar, K. Grela and M. Mauduit, Dalton Trans., 2013, 42, 7354–7358 RSC.
- C. Torborg, G. Szczepaniak, A. Zielinski, M. Malinska, K. Wozniak and K. Grela, Chem. Commun., 2013, 49, 3188–3190 RSC.
- O. Ablialimov, M. Kędziorek, M. Malińska, K. Woźniak and K. Grela, Organometallics, 2014, 33, 2160–2171 CrossRef CAS.
- A. Kajetanowicz, J. Czaban, G. R. Krishnan, M. Malińska, K. Woźniak, H. Siddique, L. G. Peeva, A. G. Livingston and K. Grela, ChemSusChem, 2013, 6, 182–192 CrossRef CAS PubMed.
- A. Ahmadi, C. McBride, J. J. Freire, A. Kajetanowicz, J. Czaban and K. Grela, J. Phys. Chem. A, 2011, 115, 12017–12024 CrossRef CAS PubMed.
- X. Bantreil, T. E. Schmid, R. A. M. Randall, A. M. Z. Slawin and C. S. J. Cazin, Chem. Commun., 2010, 46, 7115–7117 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cy02623k |
| This journal is © The Royal Society of Chemistry 2017 |