Low-bandgap donor–acceptor polymers for photodetectors with photoresponsivity from 300 nm to 1600 nm†
Jinfeng
Han
ab,
Ji
Qi
ab,
Xiuping
Zheng
ab,
Yukun
Wang
ab,
Liuyong
Hu
ab,
Chang
Guo
c,
Yang
Wang
d,
Yuning
Li
c,
Dongge
Ma
a,
Wenqiang
Qiao
*a and
Zhi Yuan
Wang
*ae
aState Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China
bUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
cDepartment of Chemical Engineering and Waterloo Institute for Nanotechnology, University of Waterloo, 200 University Avenue West, Waterloo, Ontario N2L 3G1, Canada
dThe College of Chemistry and Molecular Engineering, Center of Computational Chemistry, Zhengzhou University, Zhengzhou 450001, P. R. China
eDepartment of Chemistry, Carleton University, 1125 Colonel By Drive, Ottawa, Ontario K1S 5B, Canada. E-mail: wayne_wang@carleton.ca
First published on 25th November 2016
Abstract
A novel donor unit dithienobenzotrithiophen (DTBTT) was designed and utilized in the synthesis of a series of donor–acceptor (D–A) polymers with the thienoisoindigo-based acceptor. The relationships between the structure of polymers and the absorption spectra, electrochemistry properties, hole mobility, and photovoltaic properties of these polymers have been studied. The results showed that low-bandgap D–A polymers containing DTBTT are suitable for use in broadband polymer photodetectors. Polymer photodetectors exhibited a specific detectivity (D*) in the order of 1012 Jones, low dark current and photoresponsivity at the wavelengths from 300 to 1600 nm.
Introduction
Donor–acceptor (D–A) conjugated polymers that have high photoresponsivity in a wide spectral range have emerged in recent research as alternatives to inorganic semiconductors (e.g., Si and InGaAs) for photodetector applications.1–5 At present, one of the goals in the field of broadband photodetectors is to develop bulk-heterojunction polymer photodetectors (PPDs) that perform equally well with or better than silicon or InGaAs photodetectors, particularly showing a specific detectivity (D*) over 1012 Jones with high responsivity at wavelengths ranging from 300 nm to 1600 nm or beyond. Such polymers should have the appropriate energy level in order to facilitate the generation of excitons, separation at heterojunctions and also a high mobility to aid the exciton migration. In addition, the processability and compatibility with a receptor material such as phenyl-C61-butyric acid methyl ester (PC61BM) should be considered. To date, only a few organic and polymer photodetectors with photoresponsivity over 1200 nm are reported,6–8 although a large number of D–A conjugated polymers are available.9–18A number of electron-donating and electron-withdrawing molecules are known to be promising building blocks for high-mobility low-bandgap polymers.19–21 For example, benzotrithiophene (BTT) is a planar, electron-rich building block and has been used to construct low-bandgap conjugated polymers for use in organic thin film transistors (OTFTs) and polymer solar cells (PSCs).22–25 These polymers exhibit good film-forming properties and excellent compatibility with PC61BM, but their carrier mobility and photovoltaic performance still need to be improved.26,27 A previous study reports that a large π-conjugated system with fused aromatic rings favors the improvement of the photovoltaic properties of PSCs as a result of an increase of the short-circuit current and photo-responsivity.26 By fusing thiophene with benzodithiophene (BDT), Wu et al. obtained a dithienobenzodithiophene (DTBDT)-based polymer with improved photovoltaic properties in comparison with the BDT-based polymer, presumably attributed to the more ordered molecular packing and strong interchain interaction of the DTBDT moiety.28
Accordingly, by fusing the BTT core with thiophene, the resulting larger disc-like dithienobenzotrithiophene (DTBTT) can be deemed as a new electron-rich building block. Conceivably, the D–A conjugated polymers consisting of DTBTT and a suitable acceptor unit would have broad absorption and other desirable properties suitable for PPD applications. As an acceptor building block, thienoisoindigo (TII) is known to be strongly electron-withdrawing, which enables the formation of conjugated polymers with broad absorption and high hole mobility (14.4 cm2 V−1 s−1),29 although the TII-containing polymers still need further optimization on molecular structures and film morphology in order to improve their photovoltaic performance.30,31 In this contribution, by employing the disc-like BTT and its larger analogue DTBTT as the donor units and TII as an acceptor unit, four D–A low-bandgap polymers were synthesized and used in polymer photodetectors. The polymer photodetectors exhibited photoresponsivity in the spectral region of 300–1600 nm and a specific detectivity of ∼1011 Jones in the spectral region of 300–1300 nm.
Experimental
Four monomers, TII-2Br, TII-2T-2Br, BTT-2Sn and DTBTT-2Sn (Fig. 1), were used to make D–A polymers, namely PBT, PBTt, PDT and PDTt. By design, these polymers are structurally related; PBT and PBTt contain the same BTT donor and TII acceptor units and the latter have the extra thiophene bridging units. Similarly, a pair of PDT and PDTt is different from the extra thiophene bridging units and has a disk-like DTBTT that is larger than the BTT in other pairs of polymers.The Stille cross-coupling polymerization was carried out in chlorobenzene at 120 °C for 72 h and the polymers were obtained in 82–88% yields.32 All the polymers are of high molecular weights, film-forming and thermally stable with the onset decomposition temperatures above 340 °C (Table 1), as assessed by thermogravimetry (Fig. S2, ESI†). These polymers are soluble and are mixed well with PC61BM in chlorobenzene at 60 °C.
| Polymer | M n/Mwa | PDI | T d | λ max (nm) | E opg (eV) | E ECg (eV) | LUMOc (eV) | HOMOc (eV) | |
|---|---|---|---|---|---|---|---|---|---|
| PhCl | Film | ||||||||
| a By GPC with polystyrene standards. b Onset temperatures for 5% weight loss of nitrogen by TGA. c Calculated using equations EHOMO = −e(Eoxon + 4.40) and ELUMO = −e(Eredon + 4.40). | |||||||||
| PBT | 11.0/22.0 | 2.2 | 394 | 848 | 848 | 1.00 | 1.76 | −3.67 | −5.43 |
| PBTt | 11.3/23.8 | 1.9 | 342 | 770 | 783 | 1.26 | 1.53 | −3.73 | −5.26 |
| PDT | 73.4/132.0 | 1.8 | 400 | 866 | 879 | 0.92 | 1.28 | −3.65 | −4.93 |
| PDTt | 35.8/71.3 | 2.0 | 408 | 813 | 852 | 1.14 | 1.24 | −3.73 | −4.97 |
All the chemicals and reagents were obtained from commercial sources and used without further purification. The solvents were purified by distillation. All the reactions and operations were carried out under argon by employing the standard Schlenk techniques. The synthetic procedures are found in the ESI.†
NMR spectra were recorded on a Bruker AV400 NMR spectrometer. High-resolution matrix-assisted laser desorption ionization time-of-flight mass spectra were recorded on a Bruker Daltonics Autoflex III TOF/TOF. Thermogravimetry was done on a PerkinElmer Pyris Diamond TG instrument under continuous nitrogen flow from 50 to 800 °C at a heating rate of 10 °C min−1. Gel permeation chromatography was performed on a Waters 2414 system with chloroform as an eluent and polystyrene as a standard at room temperature. Cyclic voltammetry was done on a CHI660b electrochemical workstation in a solution of n-Bu4NPF6 in dry acetonitrile (0.1 M) with the scan rate of 50 mV s−1 at room temperature under an Ar atmosphere. The absorption spectra of polymers were recorded on a Shimadzu UV3600 spectrophotometer. Out-of-plane GIXRD profiles were obtained using a Bruker D8 Discover reflector and in-plane GIXRD profiles were obtained using a Rigaku SmartLab X-ray diffractometer. AFM images were obtained using an SPI3800N AFM (Seiko Instruments). TEM images were obtained on a JEOL JEM-1011 transmission electron microscope operated at an accelerating voltage of 100 kV with a camera length of 100 cm.
The mixture of the polymer (3 mg) and PC61BM with a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was dissolved in chlorobenzene at a concentration of 18 mg mL−1 and stirred at 80 °C for 4 h under N2. The ITO-coated glass was cleaned and treated with the plasma of oxygen. PEDOT:PSS was spin-coated onto the ITO surface at 3000 rpm for 60 s to obtain a thin film of 30 nm and dried at 110 °C for 30 min. The active layer was spin coated onto the PEDOT:PSS film at 1000 rpm for 60 s under nitrogen in a glovebox. Finally, thin layers of BCP (10 nm) and Al (120 nm) were deposited using thermal evaporation in a vacuum at a base pressure of <4 × 10−4 Pa. The effective area of every device is 16 mm2. The measurement of EQE was done on a system from Beijing 7-Star Optical Instruments Co., Ltd. The data of dark current density–voltage were obtained using a Keithley 236 Source Measure Unit.
2 was dissolved in chlorobenzene at a concentration of 18 mg mL−1 and stirred at 80 °C for 4 h under N2. The ITO-coated glass was cleaned and treated with the plasma of oxygen. PEDOT:PSS was spin-coated onto the ITO surface at 3000 rpm for 60 s to obtain a thin film of 30 nm and dried at 110 °C for 30 min. The active layer was spin coated onto the PEDOT:PSS film at 1000 rpm for 60 s under nitrogen in a glovebox. Finally, thin layers of BCP (10 nm) and Al (120 nm) were deposited using thermal evaporation in a vacuum at a base pressure of <4 × 10−4 Pa. The effective area of every device is 16 mm2. The measurement of EQE was done on a system from Beijing 7-Star Optical Instruments Co., Ltd. The data of dark current density–voltage were obtained using a Keithley 236 Source Measure Unit.
Results and discussion
Calculations of the segments of these four polymers indicate that the main chains of PBT and PBTt adopt a zigzag conformation with twist angles of 44° and 45°, respectively (Fig. 2). Thus, it is reasonable to expect that these two polymers are difficult to form crystalline domains in the solid state.28 In comparison, the backbone of polymers PDT and PDTt is more linear with a much smaller bent angle of 14–15° (Fig. 2). Therefore, the donor unit DTBTT seems to enable a linear main chain in the polymer backbone for closer interchain packing and also stronger π–π interchain interactions, which implies that PDT and PDTt would have more desirable film morphology than PBT and PBTt for PPD applications.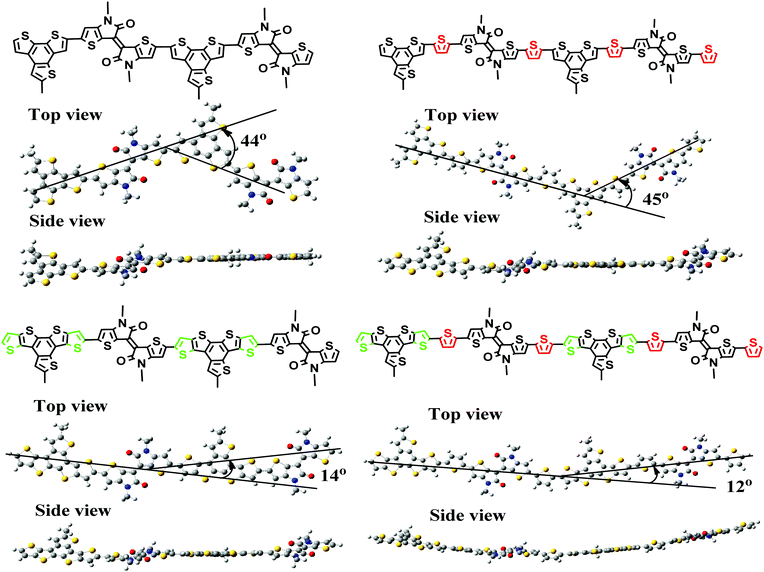 | ||
| Fig. 2 The conformations of backbone segments of each of the polymers as calculated by density functional theory (DFT) at the B3LYP/6-31G(d,p) level. | ||
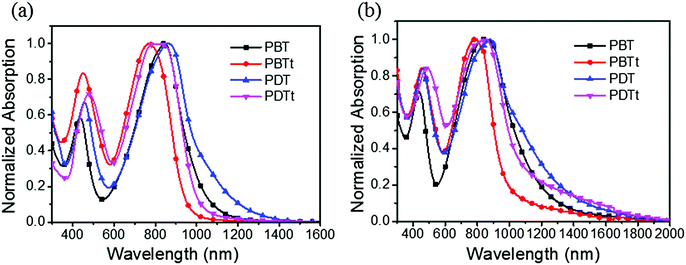
Due to the presence of a strong electron-withdrawing TII unit, all the polymers absorb broadly in the UV-Vis-NIR spectral region and display a broad and intense charge-transfer peak from 600 nm to 1340 nm (Fig. 3). Accordingly, optical band gaps (Eoptg) of PBT, PBTt, PDT and PDTt are 1.00, 1.26, 0.92 and 1.14 eV, respectively. As shown in Table 1 and Fig. S2 (ESI†), the maximal absorption of PBT, PBTt, PDT and PDTt in a film shifts at the wavelengths of 0, 13, 13 and 39 nm compared with that of chlorobenzene, respectively. A larger redshift in the solid state for DTBTT-containing polymers can be attributed to the more ordered and tight packing of polymer chains brought by the presence of a large disk-like DTBTT and linear polymer chains as predicated by calculations (Fig. 2).33
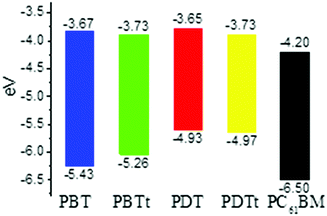
To estimate the energy levels and bandgaps of the polymer films, cyclic voltammetry (CV) of four polymers was done in 0.1 M n-Bu4NPF6 in acetonitrile (Fig. S3, ESI†). From the onset oxidation and reduction potentials, the HOMO/LUMO energy levels were calculated to be −5.43/−3.67, −5.26/−3.73, −4.93/−3.65 and −4.97/−3.73 eV, corresponding to the bandgaps of 1.76, 1.53, 1.28 and 1.24 eV, for PBT, PBTt, PDT and PDTt, respectively (Table 1 and Fig. 4). It is worth noting that the relatively narrow bandgaps of PDT and PDTt in comparison with PBT and PBTt are basically a net result of the increased HOMO levels due to the presence of a large DTBTT unit. As a result, by replacing BTT with DTBTT, the energy offsets between the HOMO energy levels of the DTBTT-containing polymers and PC61BM become larger, leading to a better charge separation between polymers and PC61BM (Fig. 4).
To study the hole-transporting properties of these polymers, the bottom-gate bottom-contact transistor devices were fabricated (Fig. S4, ESI†). The polymer films were obtained by spin-coating the polymer solution in chlorobenzene (5 mg mL−1) on the substrate at 3000 rpm for 60 s followed by subsequent annealing at 200 °C for 1 h in nitrogen. Both PBT and PBTt did not exhibit any transistor characteristics, possibly due to the poor film quality. Polymer PDT was determined to have a hole mobility of 3.5 × 10−4 cm2 V−1 s−1. Polymer PDTt exhibited a higher hole mobility of 2.2 × 10−2 cm2 V−1 s−1, which could be attributed to the presence of an extra thiophene linkage and a slightly planar geometry along the polymer main chain.
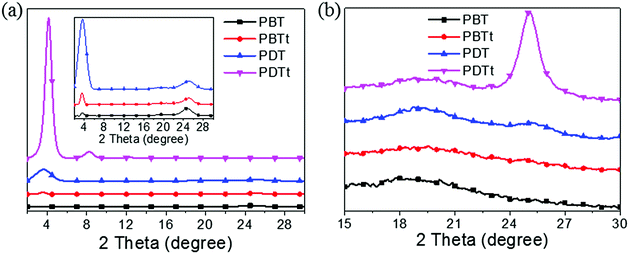
X-ray diffraction (XRD) measurement was done to further reveal the molecular packing. As shown in Fig. 5, the (100) peaks of PBT, PBTt, PDT and PDTt are 3.69°, 3.69°, 3.64° and 4.15°, respectively, corresponding to d-spacing values of 2.39, 2.39, 2.43 and 2.13 nm. The intensity of the (100) diffraction of PDTt is the most intense compared to other polymers, and the (200), (300) and (400) diffractions can also be detected at 8.30°, 12.43° and 16.73° in the out-of-plane profile. In comparison, the intensity of (100) diffractions of PDTt and PDT is more intense than those of PBTt and PBT, suggesting the higher degree of order for lamella stacking. In the out-of-plane profile, the intensity of the (010) π–π stacking peak of PDT is weaker than that of PBT and PBTt; PDTt does not have the (010) diffraction, suggesting that the large disk-like DTBTT unit promotes the edge-on orientation. The corresponding d-spacing values of π–π stacking of PBT, PBTt and PDT are 3.59, 3.56 and 3.51 Å, indicating that PDT has the smallest molecular-stacking distance in the out-of-plane profile. In the in-plane profile, an intense (010) π–π stacking peak is at 25.14°, corresponding to a d-spacing of 3.54 Å for PDTt; a weak π–π stacking peak is at 25.32° for PDT, corresponding to a d-spacing of 3.51 Å, which are consistent with their hole-transporting properties.
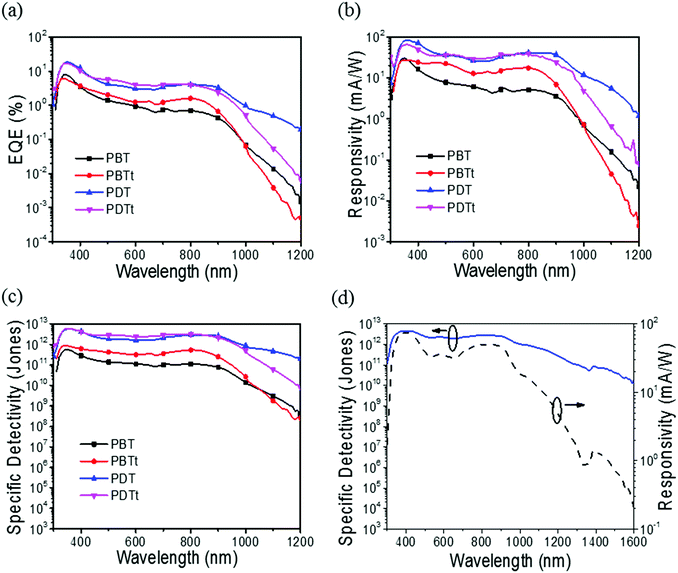
Polymer photodetectors were fabricated using the synthesized polymer as a donor material and PC61BM as an acceptor with a device architecture of ITO/PEDOT:PSS/active layer/BCP/Al. 1,8-Diiodooctane (DIO) (3% by volume) was used as an additive in the solvent to induce crystallinity and phase separation in the films.34–37 2,9-Dimethyl-4,7-diphenyl-1,10-phenanthroline (BCP) was vacuum deposited on top of the active layer.9 Since the dark current of PPDs is one of the most important parameters that determines the device performance, the thin layer of BCP is used to effectively block the hole injection from the cathode to the active layer.38 As shown in Fig. 6, the EQE values of the PPDs based on PDT and PDTt are nearly three times larger than those of PBT and PBTt under a bias of −0.1 V, suggesting an improved photovoltaic performance,29 possibly due to more effective carrier transport in the active layer. The current density–voltage (J–V) characteristics of all the PPDs are shown in Fig. S5 (ESI†). If the short noise of dark current is assumed as the main contributor to the noise,39 the specific detectivity of PPDs can be expressed as:
| D* = R/(2qJd)1/2 = (Jph/Llight)/(2qJd)1/2 (Jones) |
| Polymer | J d (A cm−2) | R 900nm (mA W−1) | D 900nm* (Jones) | |||
|---|---|---|---|---|---|---|
| −0.1 V | −1.0 V | −0.1 V | −1.0 V | −0.1 V | −1.0 V | |
| PBT | 5.08 × 10−9 | 1.50 × 10−7 | 0.3 | 3.6 | 7.7 × 1010 | 2.1 × 1010 |
| PBTt | 1.85 × 10−9 | 3.60 × 10−8 | 0.5 | 6.9 | 2.5 × 1011 | 8.1 × 1010 |
| PDT | 1.38 × 10−10 | 1.96 × 10−9 | 2.4 | 37.0 | 2.6 × 1012 | 1.9 × 1012 |
| PDTt | 2.06 × 10−10 | 3.30 × 10−9 | 1.7 | 24.0 | 2.1 × 1012 | 9.3 × 1011 |
It is well known that the performance of photovoltaic devices is closely related to the active layer morphology and phase separation. The phase separation domain of 10 nm is favorable for exciton dissociation and charge transport.41 To understand the effect of the film roughness and the phase separation on the photovoltaic properties and dark current of PPDs, atomic force microscopy (AFM) and transmission electron microscopy (TEM) were used to investigate the film morphology of active layers. The AFM topography and TEM images are shown in Fig. 7 and Fig. S9 (ESI†), respectively. The root mean square (RMS) roughness values of the active layers are 8.41, 1.25, 3.74 and 2.81 nm for the blends of PBT, PBTt, PDT and PDTt with PC61BM, respectively. All the polymers have phase-separated morphology with small-size domains. The PBT:PC61BM blend has a larger surface roughness and phase separation; the PDT blend exhibits a uniform fibrous structure; fibre-like aggregates can be observed on the surfaces of PBTt and PDTt blends. From the AFM phase images (Fig. S8, ESI†), the PBTt, each of the PDT and PDTt blends, exhibits a clear fibre-like structure. The TEM image reveals that the PDT blend has a small phase-separation with a size of 4–14 nm, which is deemed to be ideal for efficient exciton dissociation and explains well for the relatively high performance of the PDT-based photodetector.
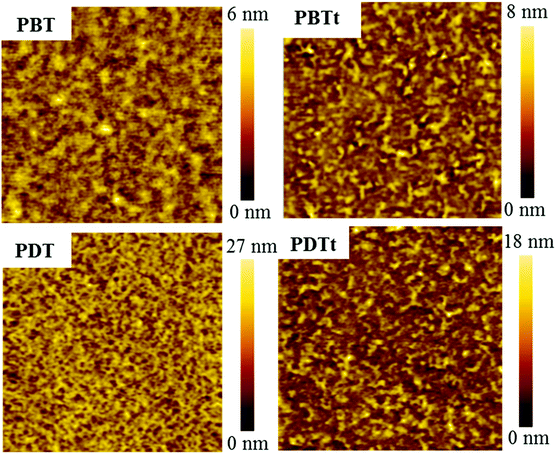 | ||
| Fig. 7 AFM height images (3 μm × 3 μm) of the BHJ active layers spin-coated on glass/ITO/PEDOT:PSS using chlorobenzene as solvent with 3% DIO. | ||
Conclusions
We found that low-bandgap polymers containing a large disk-like DTBTT unit show higher hole mobility and have better photovoltaic properties, especially in the near-infrared region. Photoresponsivity covers the spectral region of 300–1600 nm. In comparison with the low-bandgap polymers containing an analogous BTT unit, PPDs based on the DTBTT-containing polymers have a three-fold higher EQE at 900 nm under −0.1 V.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21134005, 21474102 and 21474105) and the Natural Science and Engineering Research Council of Canada.Notes and references
- X. Wang, H. Li, Z. Su, F. Fang, G. Zhang, J. Wang, B. Chu, X. Fang, Z. Wei, B. Li and W. Li, Org. Electron., 2014, 15, 2367 CrossRef CAS.
- J. Qi, X. Zhou, D. Yang, W. Qiao, D. Ma and Z. Y. Wang, Adv. Funct. Mater., 2014, 24, 7605 CrossRef CAS.
- X. Liu, H. Wang, T. Yang, W. Zhang, I. F. Hsieh, S. Z. D. Cheng and X. Gong, Org. Electron., 2012, 13, 2929 CrossRef CAS.
- W. Wang, F. Zhang, H. Bai, L. Li, M. Gao, M. Zhang and X. Zhan, Nanoscale, 2016, 8, 5578 RSC.
- L. Shen, Y. Zhang, Y. Bai, X. Zheng, Q. Wang and J. Huang, Nanoscale, 2016, 8, 12990 RSC.
- J. D. Zimmerman, V. V. Diev, K. Hanson, R. R. Lunt, E. K. Yu, M. E. Thompson and S. R. Forrest, Adv. Mater., 2010, 22, 2780 CrossRef CAS PubMed.
- M. Young, J. Suddard-Bangsund, T. J. Patrick, N. Pajares, C. J. Traverse, M. C. Barr, S. Y. Lunt and R. R. Lunt, Adv. Opt. Mater., 2016, 7, 1028–1033 CrossRef.
- E. Perzon, F. Zhang, M. Andersson, W. Mammo, O. Inganäs and M. R. Andersson, Adv. Mater., 2007, 19, 3308 CrossRef CAS.
- J. Qi, J. Han, X. Zhou, D. Yang, J. Zhang, W. Qiao, D. Ma and Z. Y. Wang, Macromolecules, 2015, 48, 3941 CrossRef CAS.
- A. J. Kronemeijer, E. Gili, M. Shahid, J. Rivnay, A. Salleo, M. Heeney and H. Sirringhaus, Adv. Mater., 2012, 24, 1558 CrossRef CAS PubMed.
- J. Lee, J.-H. Kim, B. Moon, H. G. Kim, M. Kim, J. Shin, H. Hwang and K. Cho, Macromolecules, 2015, 48, 1723 CrossRef CAS.
- H. Zhou, L. Yang and W. You, Macromolecules, 2012, 45, 607 CrossRef CAS.
- J. Zhao, Y. Li, A. Hunt, J. Zhang, H. Yao, Z. Li, J. Zhang, F. Huang, H. Ade and H. Yan, Adv. Mater., 2016, 28, 1868 CrossRef CAS PubMed.
- Y. Zhang, D. Deng, K. Lu, J. Zhang, B. Xia, Y. Zhao, J. Fang and Z. Wei, Adv. Mater., 2015, 27, 1071 CrossRef CAS PubMed.
- L. Gao, Z.-G. Zhang, L. Xue, J. Min, J. Zhang, Z. Wei and Y. Li, Adv. Mater., 2016, 28, 1884 CrossRef CAS PubMed.
- C. Mu, P. Liu, W. Ma, K. Jiang, J. Zhao, K. Zhang, Z. Chen, Z. Wei, Y. Yi, J. Wang, S. Yang, F. Huang, A. Facchetti, H. Ade and H. Yan, Adv. Mater., 2014, 26, 7224 CrossRef CAS PubMed.
- W. Li, W. S. Roelofs, M. Turbiez, M. M. Wienk and R. A. Janssen, Adv. Mater., 2014, 26, 3304 CrossRef CAS PubMed.
- W. Li, K. H. Hendriks, A. Furlan, W. S. Roelofs, S. C. Meskers, M. M. Wienk and R. A. Janssen, Adv. Mater., 2014, 26, 1565 CrossRef CAS PubMed.
- W. Zhang, Y. Han, X. Zhu, Z. Fei, Y. Feng, N. D. Treat, H. Faber, N. Stingelin, I. McCulloch, T. D. Anthopoulos and M. Heeney, Adv. Mater., 2016, 28, 3922 CrossRef CAS PubMed.
- H. J. Yun, G. B. Lee, D. S. Chung, Y. H. Kim and S. K. Kwon, Adv. Mater., 2014, 26, 6612 CrossRef CAS PubMed.
- T. Lei, X. Xia, J. Y. Wang, C. J. Liu and J. Pei, J. Am. Chem. Soc., 2014, 136, 2135 CrossRef CAS PubMed.
- C. B. Nielsen, B. C. Schroeder, A. Hadipour, B. P. Rand, S. E. Watkins and I. McCulloch, J. Mater. Chem., 2011, 21, 17642 RSC.
- X. Guo, S. R. Puniredd, M. Baumgarten, W. Pisula and K. Müllen, J. Am. Chem. Soc., 2012, 134, 8404 CrossRef CAS PubMed.
- S.-C. Lan, P.-A. Yang, M.-J. Zhu, C.-M. Yu, J.-M. Jiang and K.-H. Wei, Polym. Chem., 2013, 4, 1132 RSC.
- X. Guo, S. R. Puniredd, M. Baumgarten, W. Pisula and K. Mullen, Adv. Mater., 2013, 25, 5467 CrossRef CAS PubMed.
- L. Huo, T. Liu, X. Sun, Y. Cai, A. J. Heeger and Y. Sun, Adv. Mater., 2015, 27, 2938 CrossRef CAS PubMed.
- H. J. Son, L. Lu, W. Chen, T. Xu, T. Zheng, B. Carsten, J. Strzalka, S. B. Darling, L. X. Chen and L. Yu, Adv. Mater., 2013, 25, 838 CrossRef CAS PubMed.
- Y. Wu, Z. Li, W. Ma, Y. Huang, L. Huo, X. Guo, M. Zhang, H. Ade and J. Hou, Adv. Mater., 2013, 25, 3449 CrossRef CAS PubMed.
- G. Kim, S. J. Kang, G. K. Dutta, Y. K. Han, T. J. Shin, Y. Y. Noh and C. Yang, J. Am. Chem. Soc., 2014, 136, 9477 CrossRef CAS PubMed.
- Y. Chen, Z. Du, W. Chen, L. Han, Q. Liu, M. Sun and R. Yang, Synth. Met., 2014, 187, 24 CrossRef CAS.
- M. Ide, Y. Koizumi, A. Saeki, Y. Izumiya, H. Ohkita, S. Ito and S. Seki, J. Phys. Chem. C, 2013, 117, 26859 CAS.
- J. Yao, C. Yu, Z. Liu, H. Luo, Y. Yang, G. Zhang and D. Zhang, J. Am. Chem. Soc., 2016, 138, 173–185 CrossRef CAS PubMed.
- Q. Wu, M. Wang, X. Qiao, Y. Xiong, Y. Huang, X. Gao and H. Li, Macromolecules, 2013, 46, 3887 CrossRef CAS.
- N. A. Ran, M. Kuik, J. A. Love, C. M. Proctor, I. Nagao, G. C. Bazan and T. Q. Nguyen, Adv. Mater., 2014, 26, 7405 CrossRef CAS PubMed.
- H. Y. Chen, J. H. Hou, S. Q. Zhang, Y. Y. Liang, G. W. Yang, Y. Yang, L. P. Yu, Y. Wu and G. Li, Nat. Photonics, 2009, 3, 649 CrossRef CAS.
- Y. Y. Liang, Z. Xu, J. B. Xia, S.-T. Tsai, Y. Wu, G. Li, C. Ray and L. P. Yu, Adv. Mater., 2010, 22, 135 CrossRef PubMed.
- J. Peet, J. Y. Kim, N. E. Coates, W. L. Ma, D. Moses, A. J. Heeger and G. C. Bazan, Nat. Mater., 2007, 6, 497 CrossRef CAS PubMed.
- K. J. Baeg, M. Binda, D. Natali, M. Caironi and Y. Y. Noh, Adv. Mater., 2013, 25, 4267 CrossRef CAS PubMed.
- X. Gong, M. Tong, Y. Xia, W. Cai, J. S. Moon, Y. Cao, G. Yu, C. L. Shieh, B. Nilsson and A. J. Heeger, Science, 2009, 325, 1665 CrossRef CAS PubMed.
- Y. Yao, Y. Liang, V. Shrotriya, S. Xiao, L. Yu and Y. Yang, Adv. Mater., 2007, 19, 3979 CrossRef CAS.
- P. B. Deotare, W. Chang, E. Hontz, D. N. Congreve, L. Shi, P. D. Reusswig, B. Modtland, M. E. Bahlke, C. K. Lee, A. P. Willard, V. Bulović, T. Van Voorhis and M. A. Baldo, Nat. Mater., 2015, 14, 1130 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6tc05031j |
| This journal is © The Royal Society of Chemistry 2017 |