Open Access Article
Open Access ArticleEvolution of CsPbBr3 nanocrystals upon post-synthesis annealing under an inert atmosphere†
F.
Palazon
,
F.
Di Stasio
,
S.
Lauciello
,
R.
Krahne
,
M.
Prato
* and
L.
Manna
*
Nanochemistry Department, Istituto Italiano di Tecnologia, Via Morego 30, 16163 Genova, Italy. E-mail: mirko.prato@iit.it; liberato.manna@iit.it
First published on 8th September 2016
Abstract
Annealing a film of CsPbBr3 nanocrystals (NCs) leads to the removal of surface ligands and ripening of the NCs below 200 °C. This results in a reduced photoluminescence quantum yield due to the formation of trap states while on the other hand the conductive properties of the films are stabilized, with a significantly stronger photocurrent after annealing.
Colloidal perovskite quantum dots (QDs), and in particular colloidal CsPbBr3 nanocrystals (NCs), have been gathering considerable attention in the last couple of years,1–4 as papers in rapid succession have reported electroluminescence,5 lasing,6 fast anion-exchange reactions,7,8 fluorescence blinking and photoactivation,9 energy transfer,10 single-photon emission,11 shape control leading to bandgap tuning through quantum confinement,12,13 and stability in water.14,15 The relatively low cost of fabrication of these nanomaterials has already enabled the development of gram-scale synthesis methods16 and their implementation in different optoelectronic devices, such as LEDs.16–18 To date, the pace at which new potential applications based on CsPbBr3 NCs are being proposed is not matched by studies on how these NCs, which are much more labile than conventional semiconductor QDs, evolve under different external agents such as heat, humidity or UV exposure. Here temperature is probably the most straightforward and widespread factor to take into account. To date, the influence of temperature on the shape and optical properties of cesium lead halide NCs has been studied mainly as a parameter that is tuned during the synthesis of the NCs,19 while many more studies have been carried out on bulk perovskite films. The thermal stability of the films was found to depend on the chemical composition as well as under annealing conditions (in air, nitrogen or vacuum).20–22 Conings et al.23 demonstrated for example that annealing at 85 °C caused significant degradation of hybrid organic–inorganic perovskite thin films even under an inert atmosphere. The instability of methylammonium lead trihalide films upon annealing21 is one of the reasons that encouraged the research on the more stable, fully inorganic cesium lead trihalide (CsPbX3) counterparts.20,24 According to Sutton et al.,20 CsPbX3 thin films are characterized by both optimal crystallinity and optical performances when annealed at 350 °C. Colloidal CsPbX3 NCs are fundamentally different from their thin film counterparts, due to their much higher surface to volume ratio and their high organic content arising from the molecular ligands that coat their surface. Therefore, it is not possible to extrapolate the behaviour upon annealing of a “film” of deposited NCs on a substrate from the reported results for bulk films. The lack of extensive studies in this direction is notable, as the development of perovskite NC-based optoelectronic devices often involves heating during fabrication (e.g., lithography resist annealing)25 or during operation (i.e., Joule effect). In this work, we fill this gap by studying the effect of annealing at different temperatures (from 50 °C to 200 °C) under an inert atmosphere.
In the typical colloidal synthesis as described by Protesescu et al.2 CsPbBr3 NCs are stabilized with long chain organic ligands (oleylamine – OlAm – and oleic acid – OlAc – dissolved in octadecene – ODE –, each molecule having an 18 carbon chain). This is not anomalous in the colloidal synthesis of inorganic NCs, but what is peculiar to perovskite NCs is their instability in polar solvents, which makes cleaning of the sample rather difficult.5,6,26 Therefore, NCs are typically centrifuged without antisolvent and redispersed in toluene after discarding the supernatant.5,18 This procedure leads to a poor cleaning efficiency, leaving residues of unbound ligands as well as ODE in the final solution, as also noted by others.5,6,26 A large excess of these residues can result in a drop-cast film that remains partly fluid (gel-like) and not fully dried under ambient conditions. We show herein that annealing causes the removal of organic residues and part of the ligands at the surface of the NCs, which in turn affects the optoelectronic properties of the film. From 50 °C to 200 °C, a drop of the photoluminescence quantum yield (PLQY) is observed, accompanied by faster photoluminescence (PL) decay owing to the creation of trap states at the surface of the NCs. The CsPbBr3 NC films also demonstrated a stable electrical conductivity and increased photocurrent following the annealing steps. At high temperatures (150–200 °C) ripening of the NCs into larger crystals is observed.
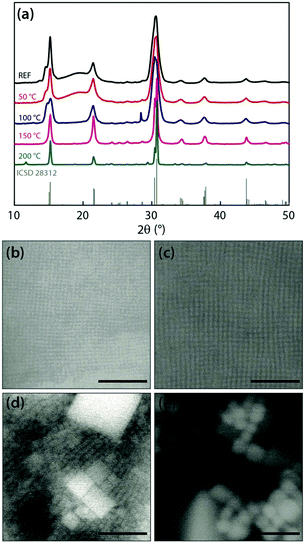
When annealing of the film is performed under ambient pressure in nitrogen, a significant portion of the organic component can be removed from the film at 100 °C. This can be seen by the disappearance of the broad feature around 20° in the XRD pattern (Fig. 1a), which is assigned to oleylamine and oleic acid27 and is further confirmed by XPS analysis (Fig. S2, ESI†). The morphology of the film remains mostly unchanged up to 100–150 °C (Fig. 1b–d) and consistent with that of a dried film of 8 nm cubes prepared at room temperature (Fig. S1, ESI†). This may suggest that the organic fraction that is removed from the film is primarily coming from free ligands and solvent residues. When annealing is performed at higher temperatures (150–200 °C), further changes become apparent. At the maximum temperature applied (200 °C) a small peak appears at 11.67°, which may be linked to the presence of CsPb2Br5 species.28 The low intensity of the peak, however, indicates that these species are residual, and the positions and relative intensities of the main peaks in the XRD patterns (Fig. 1a) remain the same, corresponding to the orthorhombic CsPbBr3 perovskite (reference pattern: ICSD 28312). Nonetheless, these are much narrower, meaning that the crystals have started ripening. Indeed, HRSEM (Fig. 1b–e) reveals much bigger crystals, with basically no trace of the original 8 nm cubes at 200 °C. We can imply that this further ripening is aided by the additional removal of surface ligands that promotes coalescence of the NCs.
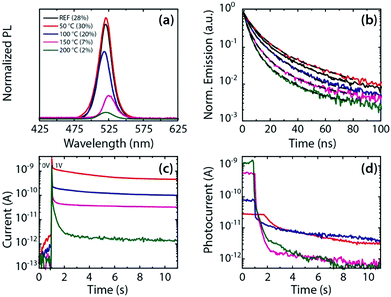
The optoelectronic properties of the films (Fig. 2) undergo significant changes already after annealing at comparatively low temperatures, i.e. below 150 °C. The PL spectra and PL decay after annealing at different temperatures are shown in Fig. 2a and b. The PL peak appears at around 521 nm with slight variations in the wavelength (<5 nm). The peak is especially red-shifted at 150 °C (525 nm), which may indicate a loss of quantum confinement as the NCs start coalescing at this temperature. However, the further blue-shift to the original value at 200 °C seems to contradict this. It is possible, however, that different transformations start taking place at this temperature (see discussion in Fig. 1a above), which presently are not completely elucidated. The significant changes appear though in PLQY and PL decay times. Both PLQY (Fig. 2a) and PL decay lifetimes (Fig. 2b and Table S1, ESI†) show a slight increase (28% to 30%, 8.6 ns to 13.9 ns, respectively) for annealing at 50 °C. The increase in PL decay time under mild annealing indicates a partial suppression of fast non-radiative channels that is beneficial for the PLQY. Annealing at 100 °C or above results in a more efficient removal of organic components (as shown in Fig. 1) and PLQY and PL lifetime drop below the values of the untreated films. PLQY drops roughly linearly from 30% (at 50 °C) to around 2% (at 200 °C), which is accompanied by a decrease in the PL lifetime from 8.57 to 4 ns at 200 °C (weighted average lifetime 〈τ〉, see Table S1, ESI†). Apart from synthetic residues, such organic components can also include the ligands that passivate the NC surface. The decrease in PLQY and PL lifetimes can be explained by the formation of trap states induced by ligand removal.29
One would expect a reduction in the organic content in a NC film to enhance its charge transport, as reported for other inorganic colloidal NCs.30 We measured the electrical current response of the NC films deposited on interdigitated Au electrode arrays before and after the annealing processes described before. Untreated films did not allow recording stable or reproducible data, and current–voltage curves measured on films annealed at low temperature showed large hysteresis and often instabilities. We therefore investigated the effect of annealing on the stability and time response of the current and photocurrent of the films. In Fig. 2c the current is recorded over time while an electrical bias voltage is abruptly switched from 0 to 1 V at t = 1 s. All current traces show a strong and fast overshoot within ms that can be attributed to the electrical measurement circuit. After that, we observe a decrease in decay time with the increasing annealing temperature that is accompanied by a reduction of the steady state current. We conclude that the annealing removes organic components from the film that contribute to conduction with a very slow time response, and that the removal is more efficient for higher annealing temperatures. The response of the photocurrent (Ilight–Idark) is shown in Fig. 2d. Here the current is measured under white light illumination that is abruptly switched off at t = 1 s. The 50 °C film exhibits only low photocurrent that decays slowly (over several seconds) once the light is switched off. Higher annealing temperatures lead to orders of magnitude higher photocurrents and significantly faster photocurrent decays. Here the ligand removal caused by higher annealing temperatures can create the additional trap states which can provide more efficient pathways for the extraction of photogenerated charges. The photocurrent decay times in the range of hundreds of milliseconds to some seconds, as observed in Fig. 2d for higher annealing temperatures, are in line with those observed from NC films of other materials.31
Conclusions
In conclusion, the evolution of the chemical and optoelectronic properties on a drop-cast film of CsPbBr3 NCs upon annealing has been described. Below 100 °C, the morphology of the initial 8 nm cubes was preserved and only the organic fraction was affected. Here, bound and unbound ligands still present in the pristine film from colloidal synthesis were removed upon annealing. Annealing above 150 °C led to the coalescence of NCs, with preservation of their orthorhombic CsPbBr3 phase. These structural and chemical transformations resulted in drastic changes in the optoelectronic properties of the drop-cast film. At 50 °C, PLQY increased from 28% to 30% demonstrating a positive effect from the treatment. However, at higher temperatures PLQY and PL decay times dropped significantly (2% at 200 °C). This is related to the creation of trap states at the surface of the NCs, originated by the stripping of the ligands. Untreated films manifested unstable and irreproducible conductivity, and annealing resulted in a more stable current with lower magnitude. The photocurrent of the films was increased by more than one order of magnitude using the annealing treatment. Our findings show that annealing under an inert atmosphere improves the performance of CsPbBr3 NCs for optoelectronic applications.Acknowledgements
The research leading to these results has received funding from the 7th European Community Framework Programme under grant agreement no. 614897 (ERC Consolidator Grant “TRANS-NANO”).Notes and references
- L. C. Schmidt, A. Pertegas, S. Gonzalez-Carrero, O. Malinkiewicz, S. Agouram, G. Minguez Espallargas, H. J. Bolink, R. E. Galian and J. Perez-Prieto, J. Am. Chem. Soc., 2014, 136, 850–853 CrossRef CAS PubMed.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15, 3692–3696 CrossRef CAS PubMed.
- G. Longo, A. Pertegás, L. Martínez-Sarti, M. Sessolo and H. J. Bolink, J. Mater. Chem. C, 2015, 3, 11286–11289 RSC.
- S. Bai, Z. Yuan and F. Gao, J. Mater. Chem. C, 2016, 4, 3898–3904 RSC.
- X. Zhang, H. Lin, H. Huang, C. J. Reckmeier, Y. Zhang, W. C. Choy and A. L. Rogach, Nano Lett., 2016, 16, 1415–1420 CrossRef CAS PubMed.
- S. Yakunin, L. Protesescu, F. Krieg, M. I. Bodnarchuk, G. Nedelcu, M. Humer, G. De Luca, M. Fiebig, W. Heiss and M. V. Kovalenko, Nat. Commun., 2015, 6, 8056 CrossRef CAS PubMed.
- Q. A. Akkerman, V. D'Innocenzo, S. Accornero, A. Scarpellini, A. Petrozza, M. Prato and L. Manna, J. Am. Chem. Soc., 2015, 137, 10276–10281 CrossRef CAS PubMed.
- G. Nedelcu, L. Protesescu, S. Yakunin, M. I. Bodnarchuk, M. J. Grotevent and M. V. Kovalenko, Nano Lett., 2015, 15, 5635–5640 CrossRef CAS PubMed.
- S. Seth, N. Mondal, S. Patra and A. Samanta, J. Phys. Chem. Lett., 2016, 7, 266–271 CrossRef CAS PubMed.
- C. de Weerd, L. Gomez, H. Zhang, W. J. Buma, G. Nedelcu, M. V. Kovalenko and T. Gregorkiewicz, J. Phys. Chem. C, 2016, 120, 13310–13315 CAS.
- Y. S. Park, S. Guo, N. S. Makarov and V. I. Klimov, ACS Nano, 2015, 9, 10386–10393 CrossRef CAS PubMed.
- Q. A. Akkerman, S. G. Motti, A. R. Srimath Kandada, E. Mosconi, V. D'Innocenzo, G. Bertoni, S. Marras, B. A. Kamino, L. Miranda, F. De Angelis, A. Petrozza, M. Prato and L. Manna, J. Am. Chem. Soc., 2016, 138, 1010–1016 CrossRef CAS PubMed.
- J. Shamsi, Z. Dang, P. Bianchini, C. Canale, F. D. Stasio, R. Brescia, M. Prato and L. Manna, J. Am. Chem. Soc., 2016, 138, 7240–7243 CrossRef CAS PubMed.
- H. Huang, B. Chen, Z. Wang, T. F. Hung, A. S. Susha, H. Zhong and A. L. Rogach, Chem. Sci., 2016, 7, 5699–5703 RSC.
- F. Palazon, Q. A. Akkerman, M. Prato and L. Manna, ACS Nano, 2016, 10, 1224–1230 CrossRef CAS PubMed.
- H. Huang, F. Zhao, L. Liu, F. Zhang, X. G. Wu, L. Shi, B. Zou, Q. Pei and H. Zhong, ACS Appl. Mater. Interfaces, 2015, 7, 28128–28133 Search PubMed.
- F. Palazon, F. Di Stasio, Q. A. Akkerman, R. Krahne, M. Prato and L. Manna, Chem. Mater., 2016, 28, 2902–2906 CrossRef CAS PubMed.
- X. Zhang, B. Xu, J. Zhang, Y. Gao, Y. Zheng, K. Wang and X. W. Sun, Adv. Funct. Mater., 2016, 26, 4595–4600 CrossRef CAS.
- D. Zhang, S. W. Eaton, Y. Yu, L. Dou and P. Yang, J. Am. Chem. Soc., 2015, 137, 9230–9233 CrossRef CAS PubMed.
- R. J. Sutton, G. E. Eperon, L. Miranda, E. S. Parrott, B. A. Kamino, J. B. Patel, M. T. Horantner, M. B. Johnston, A. A. Haghighirad, D. T. Moore and H. J. Snaith, Adv. Energy Mater., 2016, 6, 1502458 CrossRef.
- S. R. Raga, M.-C. Jung, M. V. Lee, M. R. Leyden, Y. Kato and Y. Qi, Chem. Mater., 2015, 27, 1597–1603 CrossRef CAS.
- F. X. Xie, D. Zhang, H. Su, X. Ren, K. S. Wong, M. Gratzel and W. C. Choy, ACS Nano, 2015, 9, 639–646 CrossRef CAS PubMed.
- B. Conings, J. Drijkoningen, N. Gauquelin, A. Babayigit, J. D'Haen, L. D'Olieslaeger, A. Ethirajan, J. Verbeeck, J. Manca, E. Mosconi, F. D. Angelis and H.-G. Boyen, Adv. Energy Mater., 2015, 5, 1500477 CrossRef.
- R. F. Service, Science, 2016, 351, 113–114 CrossRef CAS PubMed.
- P. Ramasamy, D. H. Lim, B. Kim, S. H. Lee, M. S. Lee and J. S. Lee, Chem. Commun., 2016, 52, 2067–2070 RSC.
- J. De Roo, M. Ibanez, P. Geiregat, G. Nedelcu, W. Walravens, J. Maes, J. C. Martins, I. Van Driessche, M. V. Kovalenko and Z. Hens, ACS Nano, 2016, 10, 2071–2081 CrossRef CAS PubMed.
- B. D. Chernomordik, A. E. Béland, N. D. Trejo, A. A. Gunawan, D. D. Deng, K. A. Mkhoyan and E. S. Aydil, J. Mater. Chem. A, 2014, 2, 10389 CAS.
- L. W. Kun-Hua Wang, L. Li, H.-B. Yao, H.-S. Qian and S.-H. Yu, Angew. Chem., Int. Ed., 2016, 55, 1–6 CrossRef.
- D. V. Talapin, A. L. Rogach, I. Mekis, S. Haubold, A. Kornowski, M. Haase and H. Weller, Colloids Surf., A, 2002, 202, 145–154 CrossRef CAS.
- M. Drndić, M. V. Jarosz, N. Y. Morgan, M. A. Kastner and M. G. Bawendi, J. Appl. Phys., 2002, 92, 7498–7503 CrossRef.
- G. Konstantatos, L. Levina, A. Fischer and E. H. Sargent, Nano Lett., 2008, 8, 1446–1450 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Methods, PL decay times and HRSEM characterization. See DOI: 10.1039/c6tc03342c |
| This journal is © The Royal Society of Chemistry 2016 |