Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Air-stable n-channel organic field-effect transistors based on charge-transfer complexes including dimethoxybenzothienobenzothiophene and tetracyanoquinodimethane derivatives†
Toshiki
Higashino‡
*a,
Masaki
Dogishi§
a,
Tomofumi
Kadoya¶
a,
Ryonosuke
Sato
a,
Tadashi
Kawamoto
a and
Takehiko
Mori
*ab
aDepartment of Organic and Polymeric Materials, Tokyo Institute of Technology, O-okayama, Meguro-ku, Tokyo 152-8552, Japan. E-mail: thigashino@issp.u-tokyo.ac.jp; mori.t.ae@m.titech.ac.jp
bACT-C, JST, Honcho, Kawaguchi, Saitama 332-0012, Japan
First published on 1st June 2016
Abstract
A new donor molecule 3,8-dimethoxy-[1]benzothieno[3,2-b][1]benzothiophene (DMeO-BTBT) is synthesized and the charge-transfer complexes with fluorinated 7,7,8,8-tetracyanoquinodimethane (Fn-TCNQ; n = 0, 2, and 4) are prepared. All complexes (DMeO-BTBT)(Fn-TCNQ) have mixed stack structures, and the thin-film and single-crystal organic transistors show n-channel transistor performance both in vacuum and in air even after one-year storage. Although the performance and stability of the thin-film transistors are improved according to the acceptor ability of Fn-TCNQ, all single-crystal transistors exhibit similar performance and excellent air stability, among which the (DMeO-BTBT)(F2-TCNQ) transistor exhibits the highest mobility of 0.097 cm2 V−1 s−1.
Introduction
Organic field-effect transistors (OFETs) have attracted considerable attention as an important component of flexible electronics.1–4 Among various organic semiconductors, [1]benzothieno[3,2-b][1]benzothiophene (BTBT) derivatives are known to show high field-effect mobility and excellent stability.5,6 A strong tendency to form a highly ordered herringbone packing is characteristic of the BTBT derivatives.7 In particular, C8-BTBT with octyl groups shows improved solubility in organic solvents and very high mobility even in solution-processed devices.8Recently, organic charge-transfer (CT) complexes incorporating BTBT derivatives have been reported.9–11 The complex of the unsubstituted BTBT with an octahedral anion, (BTBT)2PF6, shows as high conductivity as 1500 S cm−1 at room temperature, though this complex undergoes a metal–insulator transition at low temperatures.10 This complex consists of BTBT columns arranged orthogonally in a windmill manner, so the electronic structure is highly one-dimensional. Alkyl substituted BTBT derivatives (CnBTBT) form CT complexes with 7,7,8,8-tetracyanoquinodimethane (TCNQ) and fluoro TCNQ (Fn-TCNQ).11 Although these CT complexes have mixed stacks, the thin-film transistors show the electron mobility of the 10−2 cm2 V−1 s−1 order, and the single-crystal transistor achieves at most 0.4 cm2 V−1 s−1. Ambipolar transport has been also observed in the single-crystal transistor of the TCNQ complex.11a,12 Since mix-stacked CT complexes are composed of donor and accepter molecules, the transistors potentially exhibit p-channel, n-channel, and even ambipolar properties depending on the energy levels.13 However, many CT complexes including TCNQ show only n-channel transistor properties.11,14 Owing to the strong electron acceptor ability, single-component TCNQ shows air-stable n-channel transistor properties in the thin films and the crystals.15,16 Although the single-crystal transistors of TCNQ show high performance, the thin-film transistors are not easily obtained due to the high vapor pressure.15 Because air-stable n-channel transistor materials are still limited,17 it is interesting to investigate CT complexes as air-stable n-channel organic transistor materials.
In this work, we have investigated TCNQ complexes of BTBT with methoxy groups, 3,8-dimethoxy-BTBT (DMeO-BTBT, Scheme 1). BTBT has the highest occupied molecular orbital (HOMO) level at a very deep position (−5.65 eV).10a Accordingly, we have attempted to improve the donor ability by introducing electron donating substituents. DMeO-BTBT is combined with TCNQ derivatives, and three CT complexes, (DMeO-BTBT)(TCNQ), (DMeO-BTBT)(2,5-F2-TCNQ), and (DMeO-BTBT)(F4-TCNQ) are obtained. We have investigated the thin-film and single-crystal transistor properties.
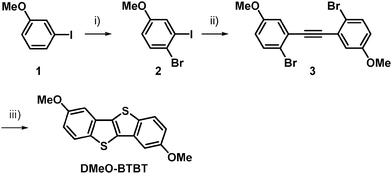 | ||
| Scheme 1 Synthesis of DMeO-BTBT: (i) NBS/DMF, 80 °C, 4 h, 99%; (ii) PdCl2(PPh3)2, CuI, DBU, H2O, TMS–acetylene/toluene, 60 °C, 18 h, 65%; (iii) CuI, Na2S·9H2O, I2/NMP, 120 °C, 48 h, 21%. | ||
Experimental
The synthesis of DMeO-BTBT is outlined in Scheme 1. Bromination of 3-iodoanisole (1) with N-bromosuccinimide (NBS) in N,N-dimethylformamide (DMF) gave 4-bromo-3-iodoanisole (2).18 Modified Sonogashira coupling of 2 with a palladium catalyst afforded 1,2-bis(2-bromo-5-methoxyphenyl)acetylene (3).19 Subsequently, heating a N-methylpyrrolidine (NMP) solution of 3 with sodium sulfide in the presence of a copper catalyst and excess iodine resulted in the ring formation to afford 3,8-dimethoxy-BTBT (DMeO-BTBT).5 Charge-transfer complexes of DMeO-BTBT with Fn-TCNQ (n = 0, 2, 4) were grown by mixing the acetonitrile solutions.Thin films of CT complexes (DMeO-BTBT)(Fn-TCNQ) were deposited by vacuum evaporation of the complexes on a tetratetracontane (TTC)-modified SiO2/Si substrate, where TTC was used as a passivation layer.20 The top-contact source and drain electrodes were fabricated by evaporating (TTF)(TCNQ) through a shadow mask with the channel length of 100 μm and the width of 1 mm.21–23 For single-crystal transistors, crystals of CT complexes were grown by the recrystallization in acetonitrile. The single-crystal transistors were fabricated by using polystyrene (PS) as a passivation layer and carbon paste as source-drain electrodes.24 The channel direction was determined by the single-crystal X-ray diffraction to be parallel to the crystal long axis corresponding to the molecular stacking (crystallographic c) axis. The transistor characteristics were measured under vacuum (10−4 Pa) and in an ambient atmosphere. The mobility was evaluated in the saturated region.
Results and discussion
Electrochemical properties and crystal structure of DMeO-BTBT
The electrochemical properties were studied by cyclic voltammetry. DMeO-BTBT shows one irreversible oxidation wave, from which the HOMO energy level is estimated to be located at −5.57 eV below the vacuum level. DMeO-BTBT is a slightly stronger donor than the unsubstituted BTBT (−5.65 eV),10a because the methoxy groups act as electron-donating groups similarly to alkyl groups; the HOMO level of 2,7-dialkyl-BTBT is located at ca. −5.5 eV.7aSingle-crystals of DMeO-BTBT for the X-ray structure analysis were grown by recrystallization from the ethyl acetate solution. The molecular packing is depicted in Fig. 1. The crystal belongs to a monoclinic system with the space group P21/c. The half molecule is crystallographically independent. Unlike the herringbone structure universally observed in BTBT derivatives, the DMeO-BTBT molecules form brickwork-like stacks similar to dimethyldicyanoquinonediimine (DMDCNQI).25 Along the c-axis, two different layers parallel to the DMeO-BTBT planes are alternately stacked with the approximate interplanar spacing of 3.53 Å. One layer consists of parallel molecules, but the molecular long axis of another layer is tilted by 70°.
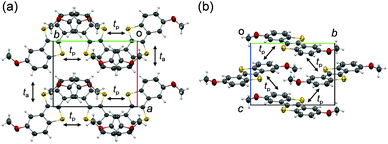 | ||
| Fig. 1 Crystal structure of DMeO-BTBT viewed along (a) the c axis, and (b) the a axis. Transfer integrals: tp = −14.2, ta = −17.0 meV. | ||
Crystal structures of the CT complexes
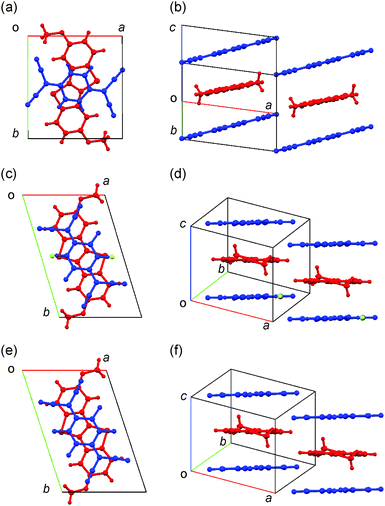
Electrochemical crystallization of DMeO-BTBT cation-radical salts with inorganic anions was attempted expecting the slightly longer DMeO-BTBT molecules alter the orthogonal molecular packing of (BTBT)2PF6, but the crystals were not obtained. Needle-like black crystals of the TCNQ complexes with the typical dimensions as large as 40 × 1 × 1 mm3 were obtained by diffusion method from the acetonitrile solutions.Single crystal X-ray structure analyses were carried out for (DMeO-BTBT)(TCNQ), (DMeO-BTBT)(F2-TCNQ) and (DMeO-BTBT)(F4-TCNQ). Table 1 lists the crystallographic data. The molecular arrangements and the intermolecular interactions are depicted in Fig. 2. All crystals belong to a triclinic system with the space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . Respective half donor and acceptor molecules are crystallographically independent; each molecule is located on an inversion center. The unit cell contains one donor and one acceptor molecules, so that the donor/acceptor ratio is 1
. Respective half donor and acceptor molecules are crystallographically independent; each molecule is located on an inversion center. The unit cell contains one donor and one acceptor molecules, so that the donor/acceptor ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. All complexes consist of mixed stacks, where the interplanar distances between the donor and acceptor planes are approximately 3.38, 3.38, and 3.36 Å, respectively; all interplanar distances are equivalent owing to the inversion center at the molecular center. In the (DMeO-BTBT)(F2-TCNQ), the fluorine atoms have positional disorder with occupancy 84% for the majority position and 16% for the minority position (Fig. S3, ESI†). The nonequivalent occupancy is related to the steric hindrance of the sulfur atoms.
1. All complexes consist of mixed stacks, where the interplanar distances between the donor and acceptor planes are approximately 3.38, 3.38, and 3.36 Å, respectively; all interplanar distances are equivalent owing to the inversion center at the molecular center. In the (DMeO-BTBT)(F2-TCNQ), the fluorine atoms have positional disorder with occupancy 84% for the majority position and 16% for the minority position (Fig. S3, ESI†). The nonequivalent occupancy is related to the steric hindrance of the sulfur atoms.
| DMeO-BTBT | (DMeO-BTBT)(TCNQ) | (DMeO-BTBT)(F2-TCNQ) | (DMeO-BTBT)(F4-TCNQ) | |
|---|---|---|---|---|
| Formula | C16H12O2S2 | C28H16N4O2S2 | C28H14F2N4O2S2 | C28H12F4N4O2S2 |
| Formula weight | 300.39 | 504.58 | 540.56 | 576.54 |
| Crystal system | Monoclinic | Triclinic | Triclinic | Triclinic |
| Space group | P21/c |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| Z | 2 | 1 | 1 | 1 |
| a (Å) | 8.286(3) | 8.693(3) | 7.777(2) | 7.971(3) |
| b (Å) | 10.555(4) | 9.371(4) | 11.695(4) | 11.720(6) |
| c (Å) | 7.814(4) | 7.302(4) | 7.117(3) | 7.066(3) |
| α (°) | 90 | 93.55(4) | 94.34(3) | 94.55(4) |
| β (°) | 95.42(3) | 97.81(4) | 105.31(3) | 105.54(3) |
| γ (°) | 90 | 89.44(3) | 72.63(2) | 71.28(3) |
| V (Å3) | 680.3(4) | 588.2(5) | 595.8(4) | 602.4(5) |
| ρ (g cm−3) | 1.466 | 1.424 | 1.506 | 1.589 |
| Total reflns. | 2339 | 4063 | 4123 | 4170 |
| Unique reflns. (Rint) | 1985 (0.0191) | 3417 (0.0215) | 3462 (0.0285) | 3503 (0.0598) |
| R 1 | 0.0327 | 0.0396 | 0.0568 | 0.0964 |
| wR2 | 0.0947 | 0.1055 | 0.1597 | 0.3494 |
| GOF | 1.018 | 1.030 | 0.955 | 1.311 |
| Temperature (K) | 298 | 298 | 298 | 298 |
In the crystal of (DMeO-BTBT)(TCNQ), the molecular long axis of the donor is approximately perpendicular to the acceptor long axis. In the crystals of (DMeO-BTBT)(F2-TCNQ) and (DMeO-BTBT)(F4-TCNQ), however, the long axes of the donor and acceptor molecules are almost parallel. Crystals of (DMeO-BTBT)(F2-TCNQ) and (DMeO-BTBT)(F4-TCNQ) are isostructural as evident from the lattice constants (Table 1). The ac lattice is approximately the same as the ab lattice of the alkyl BTBT complexes (7.8 Å × 7.1 Å),11a to indicate the structure of the core stacks are identical. In contrast, (DMeO-BTBT)(TCNQ) has an obviously different lattice.
All crystals have C–H⋯N hydrogen bonds between the Csp2–H part of DMeO-BTBT and the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N group of TCNQ derivatives. In (DMeO-BTBT)(TCNQ), the C–H⋯N distance of 2.579 Å, which is shorter than the sum of the van der Waals radii of these atoms (2.75 Å), is observed through the 4-position Csp2–H of DMeO-BTBT, leading to the orthogonal BTBT-TCNQ alignment (Fig. S4, ESI†). On the other hand, (DMeO-BTBT)(F2-TCNQ) and (DMeO-BTBT)(F4-TCNQ) has the short C–H⋯N distances (2.649 and 2.669 Å, respectively) through the 2-position Csp2–H of BTBT moiety bringing about the parallel arrangement. Furthermore, in (DMeO-BTBT)(TCNQ), a methoxy oxygen of DMeO-BTBT has a C–H⋯O interaction with the TCNQ hydrogen atom in the adjacent column (Fig. S5, ESI†). The H⋯O distance is 2.757 Å, implying the formation of the network of the very weak hydrogen bonding.26 By contrast, (DMeO-BTBT)(F2-TCNQ) and (DMeO-BTBT)(F4-TCNQ) do not have such a hydrogen bond. Therefore, the hydrogen bonding is associated with the orthogonal donor–acceptor alignment.
N group of TCNQ derivatives. In (DMeO-BTBT)(TCNQ), the C–H⋯N distance of 2.579 Å, which is shorter than the sum of the van der Waals radii of these atoms (2.75 Å), is observed through the 4-position Csp2–H of DMeO-BTBT, leading to the orthogonal BTBT-TCNQ alignment (Fig. S4, ESI†). On the other hand, (DMeO-BTBT)(F2-TCNQ) and (DMeO-BTBT)(F4-TCNQ) has the short C–H⋯N distances (2.649 and 2.669 Å, respectively) through the 2-position Csp2–H of BTBT moiety bringing about the parallel arrangement. Furthermore, in (DMeO-BTBT)(TCNQ), a methoxy oxygen of DMeO-BTBT has a C–H⋯O interaction with the TCNQ hydrogen atom in the adjacent column (Fig. S5, ESI†). The H⋯O distance is 2.757 Å, implying the formation of the network of the very weak hydrogen bonding.26 By contrast, (DMeO-BTBT)(F2-TCNQ) and (DMeO-BTBT)(F4-TCNQ) do not have such a hydrogen bond. Therefore, the hydrogen bonding is associated with the orthogonal donor–acceptor alignment.
The charge-transfer degrees estimated from the bond lengths of the TCNQ molecules (ESI†) are small, so the present TCNQ complexes are essentially regarded as neutral complexes.
The effective transfer integrals for holes and electrons (teffh and teffe) are estimated by an energy-splitting approach for the D–A–D (teffh) and A–D–A (teffe) triads (Table 2 and Fig. S6–S8, ESI†).27 The resulting values are 20–70 meV. In the TCNQ complex, teffh and teffe are comparable, whereas in the F2-TCNQ and F4-TCNQ complexes, teffe is predominant. This is attributed to the difference of the parallel and perpendicular molecular arrangements.
| Crystal | t effh (meV) | t effe (meV) |
|---|---|---|
| (DMeO-BTBT)(TCNQ) | 37 | 55 |
| (DMeO-BTBT)(F2-TCNQ) | 23 | 71 |
| (DMeO-BTBT)(F4-TCNQ) | 21 | 75 |
Thin-film properties
X-ray diffraction (XRD) patterns, and atomic force microscopy (AFM) images of the thin films deposited on the tetratetracontane (TTC)-modified Si/SiO2 substrate were obtained. As shown in Fig. 3a, the XRD patterns show diffraction peaks around 2θ = 7.9° attributed to the CT complexes (d = 11.1–11.4 Å). These d-spacings correspond to the b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α
α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) γ of the crystal lattice in (DMeO-BTBT)(F2-TCNQ) and (DMeO-BTBT)(F4-TCNQ), indicating that the crystallographic ac plane is aligned parallel to the substrate. In (DMeO-BTBT)(TCNQ), the observed d-spacing does not coincide with the crystal lattice, but is very close to the d-spacing of the other complexes. This suggests the thin-film phase is different from the crystal phase, and isostructural to the other crystals, where the donor long axis is almost parallel to the acceptor long axis. In the thin film of (DMeO-BTBT)(TCNQ), another small peak is observed at 2θ ∼ 10.3° (d = 8.6 Å). This d-spacing correspond to the a-axis of the crystal lattice, indicating the presence of a small amount of the crystal phase. (DMeO-BTBT)(F2-TCNQ) shows a diffraction peak boarder than the others as indicated by the comparatively large full width at half maximum (FWHM) in Fig. 3a. This is associated with the disordered arrangement of the F2-TCNQ complex. The small peak of (DMeO-BTBT)(TCNQ) coming from the single-crystal phase (2θ = 10.3°) also shows a large FWHM, which suggests comparatively poor crystallinity of the crystal phase in the thin films. AFM images of the evaporated thin films are shown in Fig. 3b–d. In all thin films, needle-like microcrystals cover the substrate densely. The thin film of (DMeO-BTBT)(TCNQ) shows relatively larger roughness than (DMeO-BTBT)(F2-TCNQ) and (DMeO-BTBT)(F4-TCNQ). This may be related to the presence of the two phases. In contrast, the other thin films consisting of one kind of layer structure show comparatively smooth surface.
γ of the crystal lattice in (DMeO-BTBT)(F2-TCNQ) and (DMeO-BTBT)(F4-TCNQ), indicating that the crystallographic ac plane is aligned parallel to the substrate. In (DMeO-BTBT)(TCNQ), the observed d-spacing does not coincide with the crystal lattice, but is very close to the d-spacing of the other complexes. This suggests the thin-film phase is different from the crystal phase, and isostructural to the other crystals, where the donor long axis is almost parallel to the acceptor long axis. In the thin film of (DMeO-BTBT)(TCNQ), another small peak is observed at 2θ ∼ 10.3° (d = 8.6 Å). This d-spacing correspond to the a-axis of the crystal lattice, indicating the presence of a small amount of the crystal phase. (DMeO-BTBT)(F2-TCNQ) shows a diffraction peak boarder than the others as indicated by the comparatively large full width at half maximum (FWHM) in Fig. 3a. This is associated with the disordered arrangement of the F2-TCNQ complex. The small peak of (DMeO-BTBT)(TCNQ) coming from the single-crystal phase (2θ = 10.3°) also shows a large FWHM, which suggests comparatively poor crystallinity of the crystal phase in the thin films. AFM images of the evaporated thin films are shown in Fig. 3b–d. In all thin films, needle-like microcrystals cover the substrate densely. The thin film of (DMeO-BTBT)(TCNQ) shows relatively larger roughness than (DMeO-BTBT)(F2-TCNQ) and (DMeO-BTBT)(F4-TCNQ). This may be related to the presence of the two phases. In contrast, the other thin films consisting of one kind of layer structure show comparatively smooth surface.
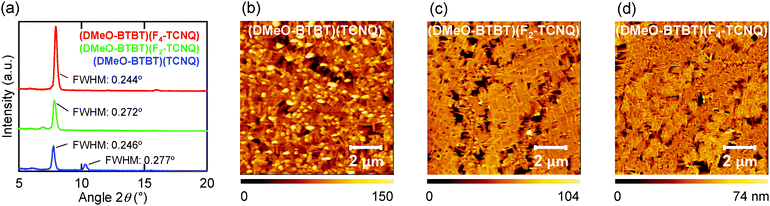 | ||
| Fig. 3 (a) XRD patterns and AFM images of (b) (DMeO-BTBT)(TCNQ), (c) (DMeO-BTBT)(F2-TCNQ), and (d) (DMeO-BTBT)(F4-TCNQ) thin films (50 nm) deposited on 20 nm TTC. | ||
Transistor properties
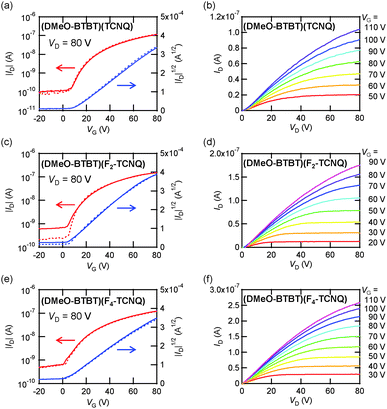
Transfer and output characteristics of the single-crystal transistors are shown in Fig. 4. The device parameters are summarized in Table 3. The CT complexes show n-channel transistor properties both in vacuum and in air. It has been generally observed that mixed-stack CT complexes containing TCNQ show only n-channel transistor properties, though ambipolar properties are reported in a few single-crystal transistors.11–14 In addition, we have to mention the comparatively weak donor ability of DMeO-BTBT (Fig. 5), where electron injection to Fn-TCNQ is preferable to hole injection to DMeO-BTBT.13c Neutral DMeO-BTBT does not show any transistor properties in the form of either single crystals or thin films. The crystal structure of DMe-BTBT (Fig. 1) is entirely different from the herringbone structure of alkyl-BTBT, and the large molecular displacement and the resulting small intermolecular overlap are responsible for the absence of the transistor properties.| Devices | Acceptors | Conditions | μ av [μmax] (cm2 V−1 s−1) | V th (V) | On/off |
|---|---|---|---|---|---|
| Thin-film | TCNQ | In vacuum | 2.1 × 10−4 [4.7 × 10−4] | 30 | 103 |
| In air | 1.2 × 10−5 [1.8 × 10−5] | 33 | 103 | ||
| F2-TCNQ | In vacuum | 6.3 × 10−4 [7.3 × 10−4] | 29 | 103 | |
| In air | 6.6 × 10−4 [8.2 × 10−4] | 48 | 103 | ||
| In air (after one year) | 1.4 × 10−3 [1.6 × 10−3] | 70 | 103 | ||
| F4-TCNQ | In vacuum | 6.7 × 10−3 [0.022] | 10 | 103 | |
| In air | 2.0 × 10−3 [2.5 × 10−3] | 27 | 103 | ||
| In air (after one year) | 2.6 × 10−3 [2.7 × 10−3] | 7 | 103 | ||
| Single-crystal | TCNQ | In vacuum | 0.016 [0.060] | 18 | 103 |
| In air | 0.015 [0.055] | 19 | 103 | ||
| In air (after one year) | 0.047 [0.060] | 15 | 103 | ||
| F2-TCNQ | In vacuum | 0.033 [0.097] | 4 | 102 | |
| In air | 0.024 [0.060] | 9 | 102 | ||
| F4-TCNQ | In vacuum | 0.018 [0.049] | −1 | 102 | |
| In air | 0.021 [0.049] | 2 | 102 | ||
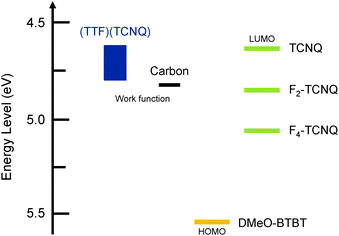 | ||
| Fig. 5 HOMO level of DMeO-BTBT, LUMO levels of TCNQ derivatives,28 and the Fermi levels of (TTF)(TCNQ),29 and carbon.30 | ||
Among the thin-film transistors, the highest electron mobility of 0.022 cm2 V−1 s−1 is observed in (DMeO-BTBT)(F4-TCNQ) in vacuum. The mobility increases in the order of TCNQ < F2-TCNQ < F4-TCNQ. This order is potentially related to the film quality (Fig. 3); the XRD intensity increases, and the AFM roughness decreases in this order. However, since the mobilities of the thin-film devices are to some extent reduced in air, we could not exclude the potential influence of the improved acceptor ability (Fig. 5).
By contrast, the single-crystal transistors do not show a systematic increase, and all complexes exhibit similar mobilities in the order of 10−2 cm2 V−1 s−1. The highest mobility is 0.097 cm2 V−1 s−1 in (DMeO-BTBT)(F2-TCNQ), but other complexes show mobilities of the same order. This value is not largely different from 0.4 cm2 V−1 s−1 reported in (CnBTBT)(TCNQ)-based single-crystal transistors.11 The transistor properties of the single-crystal transistors do not change in air. This is obvious from Fig. 4 as well as the mobility values in Table 3. The performance in air is practically unchanged even after one-year storage (Table 3 and Fig. S10, ESI†); note that the mobility values listed in Table 3 even slightly increase after one-year storage. It should be also pointed out that the threshold voltage Vth is large in the thin-film transistors (Table 3), and increases in air. In contrast, Vth is small in the single-crystal transistors, particularly in the F4-TCNQ complex. In general, it is difficult to realize air-stable n-channel organic transistors because the presence of gaseous water and oxygen reduces the performance.31 This particularly applies to thin-film transistors, and among the present combinations, even F4-TCNQ is insufficient to entirely eliminate the drop of the mobility and the shift of Vth in air. The present work demonstrates that the single-crystal transistors are almost free from such instabilities. This is probably because water and oxygen do not penetrate into the crystals. As a result, TCNQ is sufficient to achieve the air-stable performance.
Conclusions
We have prepared a new donor molecule DMeO-BTBT and investigated the crystal structure and transistor properties of the CT complexes (DMeO-BTBT)(Fn-TCNQ) (n = 0, 2, and 4). The thin-film and single-crystal transistors show n-channel transistor properties even in air. Although the performance and stability of the thin-film transistors are considerably influenced by the acceptor strength, the single-crystal transistors are particularly stable in air, and even the TCNQ complex is sufficient to achieve the excellent air stability. Since it is still difficult to realize air-stable n-channel organic transistors, the use of the CT complexes is an important strategy, where these TCNQ complexes achieve fairly good performance in spite of the mixed stack structures. The present work demonstrates, in such a case, the single-crystal transistors show remarkably improved stability in comparison with the thin-film transistors.Acknowledgements
The authors are grateful to Center for Advanced Materials Analysis, Tokyo Institute of Technology, for X-ray diffraction measurements. The authors also gratefully acknowledge Prof. H. Mori, The University of Tokyo, for the DFT calculation using the Gaussian 03 package.Notes and references
- C. D. Dimitrakopoulos and P. R. L. Malenfant, Adv. Mater., 2002, 14, 99 CrossRef CAS.
- C. Wang, H. Dong, W. Hu, Y. Liu and D. Zhu, Chem. Rev., 2012, 112, 2208 CrossRef CAS PubMed.
- A. R. Murphy and J. M. J. Frechet, Chem. Rev., 2007, 107, 1066 CrossRef CAS PubMed.
- V. Coropceanu, J. Cornil, D. A. S. Filho, Y. Olivier, R. Silbey and J. L. Brédas, Chem. Rev., 2007, 107, 926 CrossRef CAS PubMed.
- K. Takimiya, S. Shinamura, I. Osaka and E. Miyazaki, Adv. Mater., 2011, 23, 12604 CrossRef PubMed.
- (a) K. Takimiya, H. Ebata, K. Sakamoto, T. Izawa, T. Otsubo and Y. Kunugi, J. Am. Chem. Soc., 2006, 128, 12604 CrossRef CAS PubMed; (b) A. Y. Amin, A. Khassanov, K. Reuter, T. Meyer-Friedrichsen and M. Halik, J. Am. Chem. Soc., 2012, 134, 16548 CrossRef CAS PubMed; (c) H. Iino, T. Usui and J. Hanna, Nat. Commun., 2015, 6, 6828 CrossRef CAS PubMed.
- (a) H. Ebata, T. Izawa, E. Miyazaki, K. Takimiya, M. Ikeda, H. Kuwabara and T. Yui, J. Am. Chem. Soc., 2007, 129, 15732 CrossRef CAS PubMed; (b) T. Izawa, E. Miyazaki and K. Takimiya, Adv. Mater., 2008, 20, 3388 CrossRef CAS; (c) A. Kumatani, C. Liu, Y. Li, P. Darmawan, K. Takimiya, T. Minari and K. Tsukagoshi, Sci. Rep., 2012, 2, 393 Search PubMed; (d) Y. Li, C. Liu, Y. Wang, Y. Yang, X. Wang, Y. Shi and K. Tsukagoshi, AIP Adv., 2013, 3, 052123 CrossRef; (e) H. Minemawari, J. Tsutsumi, S. Inoue, T. Yamada, R. Kumai and T. Hasegawa, Appl. Phys. Express, 2014, 7, 091601 CrossRef; (f) G. Schweicher, V. Lemaur, C. Niebel, C. Ruzié, Y. Diao, O. Goto, W.-Y. Lee, Y. Kim, J.-B. Arlin, J. Karpinska, A. R. Kennedy, S. R. Parkin, Y. Olivier, S. C. B. Mannsfeld, J. Cornil, Y. H. Geerts and Z. Bao, Adv. Mater., 2015, 27, 3066 CrossRef CAS PubMed.
- (a) T. Uemura, Y. Hirose, M. Uno, K. Takimiya and J. Takeya, Appl. Phys. Express, 2009, 2, 111501 CrossRef; (b) H. Minemawari, T. Yamada, H. Matsui, J. Tsutsumi, S. Haas, R. Chiba, R. Kumai and T. Hasegawa, Nature, 2011, 475, 364 CrossRef CAS PubMed; (c) Y. Yuan, G. Giri, A. L. Ayzner, A. P. Zoombelt, S. C. B. Mannsfeld, J. Chen, D. Nordlund, M. F. Toney, J. Huang and Z. Bao, Nat. Commun., 2014, 5, 3005 Search PubMed.
- H. Méndez, G. Heimel, A. Opitz, K. Sauer, P. Barkowski, M. Oehzelt, J. Soeda, T. Okamoto, J. Takeya, J. B. Arlin, J. Y. Balandier, Y. Geerts, N. Koch and I. Salzmann, Angew. Chem., Int. Ed., 2013, 52, 7751 CrossRef PubMed.
- (a) T. Kadoya, M. Ashizawa, T. Higashino, T. Kawamoto, S. Kumeta, H. Matsumoto and T. Mori, Phys. Chem. Chem. Phys., 2013, 15, 17818 RSC; (b) Y. Kiyota, T. Kadoya, K. Yamamoto, K. Iijima, T. Higashino, T. Kawamoto, K. Takimiya and T. Mori, J. Am. Chem. Soc., 2016, 138, 3920 CrossRef CAS PubMed; (c) T. Higashino, T. Kadoya, S. Kumeta, K. Kurata, T. Kawamoto and T. Mori, Eur. J. Inorg. Chem., 2014, 3895 CrossRef CAS.
- (a) J. Tsutsumi, S. Matsuoka, S. Inoue, H. Minemawari, T. Yamada and T. Hasegawa, J. Mater. Chem. C, 2015, 3, 1976 RSC; (b) Y. Shibata, J. Tsutsumi, S. Matsuoka, K. Matsubara, Y. Yoshida, M. Chikamatsu and T. Hasegawa, Appl. Phys. Lett., 2015, 106, 143303 CrossRef.
- (a) J. Zhang, H. Geng, T. S. Virk, Y. Zhao, J. Tan, C. Di, W. Xu, K. Shingh, W. Hu, Z. Shuai, Y. Liu and D. Zhu, Adv. Mater., 2012, 24, 2603 CrossRef CAS PubMed; (b) D. Vermeulen, L. Y. Zhu, K. P. Goetz, P. Hu, H. Jiang, C. S. Day, O. D. Jurchescu, V. Coropceanu, C. Kloc and L. E. McNeil, J. Phys. Chem. C, 2014, 118, 24688 CrossRef CAS; (c) W. Zhu, Y. Yi, Y. Zhen and W. Hu, Small, 2015, 11, 2150 CrossRef CAS PubMed.
- (a) T. Hasegawa, K. Mattenberger, J. Takeya and B. Batlog, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 245115 CrossRef; (b) Y. Takahashi, T. Hasegawa, Y. Abe, Y. Tokura, K. Nishimura and G. Saito, Appl. Phys. Lett., 2005, 86, 063504 CrossRef; (c) Y. Takahashi, T. Hasegawa, Y. Abe, Y. Tokura and G. Saito, Appl. Phys. Lett., 2006, 88, 073504 CrossRef.
- (a) K. Shibata, K. Ishikawa, H. Takezoe, H. Wada and T. Mori, Appl. Phys. Lett., 2008, 92, 023305 CrossRef; (b) J. Inoue, M. Kanno, M. Ashizawa, C. Seo, A. Tanioka and T. Mori, Chem. Lett., 2010, 39, 538 CrossRef CAS.
- T. Takahashi, S. Tamura, Y. Akiyama, T. Kadoya, T. Kawamoto and T. Mori, Appl. Phys. Express, 2012, 5, 061601 CrossRef.
- (a) E. Menard, V. Podzorov, S. H. Hur, A. Gaur, M. E. Gershenson and J. A. Rogers, Adv. Mater., 2004, 16, 2097 CrossRef CAS; (b) M. Yamagishi, Y. Tominari, T. Uemura and J. Takeya, Appl. Phys. Lett., 2009, 94, 053305 CrossRef; (c) Y. Krupskaya, M. Gibertini, N. Marzari and A. F. Morpurgo, Adv. Mater., 2015, 27, 2453 CrossRef CAS PubMed.
- (a) Y. Yamashita, Chem. Lett., 2009, 38, 870 CrossRef CAS; (b) Y. Wen and Y. Liu, Adv. Mater., 2010, 22, 1331 CrossRef CAS PubMed; (c) X. Gao, C. Di, Y. Hu, X. Yang, H. Fan, F. Zhang, Y. Liu, H. Li and D. Zhu, J. Am. Chem. Soc., 2010, 132, 3697 CrossRef CAS PubMed; (d) H. Usta, A. Facchetti and T. J. Marks, Acc. Chem. Res., 2011, 44, 501 CrossRef CAS PubMed; (e) X. Zhan, A. Facchetti, S. Barlow, T. J. Marks, M. A. Ratner, M. R. Wasielewki and S. R. Marder, Adv. Mater., 2011, 23, 268 CrossRef CAS PubMed; (f) Y. Hu, X. Gao, C. Di, X. Yang, F. Zhang, Y. Liu, H. Li and D. Zhu, Chem. Mater., 2011, 23, 1204 CrossRef CAS; (g) L. Tan, Y. Guo, G. Zhang, Y. Yang, D. Zhang, G. Yu, W. Xu and Y. Liu, J. Mater. Chem., 2011, 21, 18042 RSC; (h) Y. Qiao, Y. Guo, C. Yu, F. Zhang, W. Xu, Y. Liu and D. Zhu, J. Am. Chem. Soc., 2012, 134, 4084 CrossRef CAS PubMed; (i) F. Zhang, Y. Hu, T. Schuettfort, C. Di, X. Gao, C. R. McNeill, L. Thomsen, S. C. B. Mannsfeld, W. Yuan, H. Sirringhaus and D. Zhu, J. Am. Chem. Soc., 2013, 135, 2338 CrossRef CAS PubMed; (j) X. Gao and Y. Hu, J. Mater. Chem. C, 2014, 2, 3099 RSC; (k) A. Filatre-Furcate, T. Higashino, D. Lorcy and T. Mori, J. Mater. Chem. C, 2015, 3, 3569 RSC.
- B. L. Flynn, P. Verdier-Pinard and E. Hamel, Org. Lett., 2001, 5, 651 CrossRef.
- M. J. Mio, L. C. Kopel, J. B. Braun, T. L. Gadzikwa, K. L. Hull, R. G. Brisbois, C. J. Markworth and P. A. Grieco, Org. Lett., 2002, 19, 3199 CrossRef.
- (a) M. Kraus, S. Richler, A. Opitz, W. Brütting, S. Haas, T. Hasegawa, A. Hinderhofer and F. Schreiber, J. Appl. Phys., 2010, 107, 094503 CrossRef; (b) M. Kraus, S. Haug, W. Brütting and A. Opitz, Org. Electron., 2011, 12, 731 CrossRef CAS.
- L. B. Coleman, M. J. Cohen, D. J. Sandman, F. G. Yamagishi, A. F. Gartio and A. J. Heeger, Solid State Commun., 1973, 12, 1125 CrossRef CAS.
- (a) K. Shibata, H. Wada, K. Ishikawa, H. Takezoe and T. Mori, Appl. Phys. Lett., 2007, 90, 193509 CrossRef; (b) T. Kadoya, D. de Caro, K. Jacob, C. Faulmann, L. Valade and T. Mori, J. Mater. Chem., 2011, 21, 18421 RSC.
- (a) S. Tamura, T. Kadoya, T. Kawamoto and T. Mori, Appl. Phys. Lett., 2013, 102, 063305 CrossRef; (b) S. Tamura, T. Kadoya and T. Mori, Appl. Phys. Lett., 2014, 105, 023301 CrossRef.
- J. Cho, T. Higashino and T. Mori, Appl. Phys. Lett., 2015, 106, 193303 CrossRef.
- A. Aumuller, P. Erk, S. Hunig, E. Hadicke, K. Peters and H. G. von Schnering, Chem. Ber., 1991, 124, 2001 CrossRef.
- (a) J. R. Desiraju, Acc. Chem. Res., 1991, 24, 290 CrossRef; (b) T. Higashino, S. Kumeta, S. Tamura, Y. Ando, K. Ohmori, K. Suzuki and T. Mori, J. Mater. Chem. C, 2015, 3, 1588 RSC.
- (a) L. Zhu, Y. Yi, Y. Li, E.-G. Kim, V. Coropceanu and J.-L. Brédas, J. Am. Chem. Soc., 2012, 134, 2340 CrossRef CAS PubMed; (b) L. Zhu, Y. Yi, A. Fonari, N. S. Corbin, V. Coropceanu and J.-L. Brédas, J. Phys. Chem. C, 2014, 118, 14150 CrossRef CAS; (c) H. Geng, X. Zheng, Z. Shuai, L. Zhu and Y. Yi, Adv. Mater., 2015, 27, 1443 CrossRef CAS PubMed.
- S.-S. Pac and G. Saito, J. Solid State Chem., 2002, 168, 486 CrossRef CAS.
- (a) C. R. Newman, C. D. Frisbie, D. A. da Silva Filho, J.-L. Bredas, P. C. Ewbank and K. R. Mann, Chem. Mater., 2004, 16, 4436 CrossRef CAS; (b) T. Mori, Chem. Lett., 2011, 40, 428 CrossRef CAS; (c) D. de Caro, K. Jacob, H. Hahioui, C. Faulmann, L. Valade, T. Kadoya, T. Mori, J. Fraxedas and L. Viau, New J. Chem., 2011, 35, 1315 RSC.
- M. Shiraishi and M. Ata, Carbon, 2001, 39, 1913 CrossRef CAS.
- (a) D. M. de Leeuw, M. M. J. Simenon, A. R. Brown and R. E. F. Einerhand, Synth. Met., 1997, 87, 53 CrossRef CAS; (b) D. Shukla, S. F. Nelson, D. C. Freeman, M. Rajeswaran, W. G. Ahearn, D. M. Meyer and J. T. Carey, Chem. Mater., 2008, 20, 7486 CrossRef CAS; (c) Y. Ie, M. Ueta, M. Nitani, N. Tohnai, M. Miyata, H. Tada and Y. Aso, Chem. Mater., 2012, 24, 3285 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: CCDC contain the supplementary crystallographic information for DMeO-BTBT and its CT complexes. Additional information for synthesis, optical and electrochemical properties, crystal structures, band calculations, degree of charge transfer, device fabrication, and thin-film transistor characteristics in vacuum and in air together with those after one-year storage. CCDC 1409469–1409472. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6tc01532h |
| ‡ Present address: The Institute for Solid State Physics, The University of Tokyo, Kashiwa, Chiba 277-8581, Japan. |
| § Present address: Imaging Science and Engineering Laboratory, Tokyo Institute of Technology, Yokohama, Kanagawa 226-8503, Japan. |
| ¶ Present address: Graduate School of Material Science, University of Hyogo, Kamigori, Hyogo 678-1205, Japan. |
| This journal is © The Royal Society of Chemistry 2016 |