Pyridyl-substituted anthracene derivatives with solid-state emission and charge transport properties†
Abstract
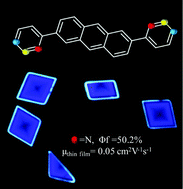
We report here a systematic study of the solid-state packing, optoelectronic properties and organic field-effect transistor properties of three isomeric 2,6-di-pyridyl anthracene derivatives (1a, 1b and 1c). Very different solid-state physicochemical behaviours were found as a result of the subtle change in the substitution site, especially in the solid-state emission and charge transport properties. For 1a and 1b, which adopted H-like aggregates in the solid state, a bright blue emission with fluorescent quantum efficiencies (ΦF) of 50.2 and 17.5% and thin film mobilities of 0.05 and 10−5 cm2 V−1 s−1, respectively, were obtained. The inferior mobility of 1b might be caused by the larger torsion and herringbone angle in the solid-state packing. A green emission with ΦF of 16% was obtained for single crystals of 1c. No charge transport property was observed for 1c, which might be related to the unfavourable solid-state packing and poor film morphology.

 Please wait while we load your content...
Please wait while we load your content...