A novel donor–acceptor carbazole and benzothiadiazole material for deep red and infrared emitting applications†
Abstract
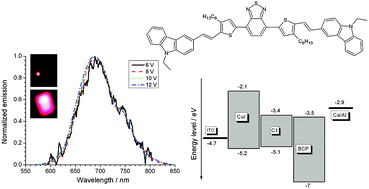
A novel organic material (C1) with the structure D–π–A–π–D was synthesised and characterised. Carbazole was utilised as the electron donor and benzothiadiazole as the electron acceptor unit. The electrochemical, optical and electronic properties of the synthesised compound were studied. Compound C1 exhibits absorption in the visible and ultraviolet range with a high molar absorption coefficient. A strong solvatochromic effect was observed in its emission spectra. Electrochemical and spectroelectrochemical measurements were performed in order to estimate the properties of the molecule in different redox states. Electron paramagnetic resonance (EPR) measurements indicate the delocalisation of radical cations and radical anions over different moieties. Interpretations of the electrochemical and optical results are supported by DFT calculations. OLEDs based on C1 present efficient emission in red and infrared spectral ranges, with a quantum efficiency of 3.13% and a current efficiency of 6.8 cd A−1. The performance is considerably better than what has been reported for analogous devices, based on other carbazole and benzothiadiazole units.

 Please wait while we load your content...
Please wait while we load your content...