Aggregation-induced emission enhancement and mechanofluorochromic properties of α-cyanostilbene functionalized tetraphenyl imidazole derivatives†
Abstract
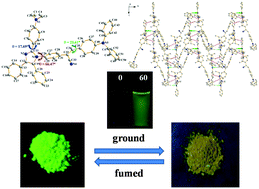
Three tetraphenyl imidazole derivatives functionalized by an α-cyanostilbene unit (3a–3c) have been designed and synthesized. A combination of the representative aggregation-induced emission enhancement fluorophore and propeller sharp tetraphenyl imidazole unit in the same molecule achieved the integration of their function. 3a–3c emitted weakly in dilute organic solvents and showed an evident solvatochromic effect caused by strong intramolecular charge transfer (ICT), which has been confirmed by density functional theory (DFT) calculations. Meanwhile, in the solid state, they exhibited obvious fluorescence and stimuli-responsive emission. The main emission peak of compound 3a was red-shifted from 519 nm to 550 nm after grinding. The reason could be explained that the destruction of the crystalline structure leads to the planarization of molecular conformation or the increase of conjugation.
- This article is part of the themed collection: Shape-Responsive Fluorophores

 Please wait while we load your content...
Please wait while we load your content...