Enhanced high temperature thermoelectric response of sulphuric acid treated conducting polymer thin films†
S. R. Sarath
Kumar‡
,
Narendra
Kurra‡
and
H. N.
Alshareef
*
Materials Science and Engineering, King Abdullah University of Science and Technology (KAUST), Thuwal-23955-6900, Saudi Arabia. E-mail: husam.alshareef@kaust.edu.sa
First published on 24th November 2015
Abstract
We report the high temperature thermoelectric properties of solution processed untreated and sulphuric acid treated poly(3,4-ethylenedioxythiophene):poly(4-styrenesulfonate) (or PEDOT:PSS) films. The acid treatment is shown to simultaneously enhance the electrical conductivity and the Seebeck coefficient of the metal-like films, resulting in a five-fold increase in the thermoelectric power factor (from 0.01 to 0.052 W m−1 K−1) at 460 K, compared to the untreated film. By using atomic force micrographs, Raman and impedance spectra and using a series heterogeneous model for electrical conductivity, we demonstrate that acid treatment results in the removal of PSS from the films, leading to the quenching of accumulated charge-induced energy barriers, facilitating metal-like conduction. The continuous removal of PSS and changes in morphology of the PEDOT grains upon acid treatment may alter the local band structure of PEDOT:PSS, in such a way as to simultaneously enhance the Seebeck coefficient.
1. Introduction
Conducting polymers (CPs), also known as synthetic metals, have become attractive due to their intriguing optical and electronic properties.1–4 Solution processability, flexibility, light-weight and cost-effectiveness are the key attributes of CPs which make them compatible in various applications.4–6 For example, CPs can be used as both active and passive materials in organic electronics6,7 and as chemical or electrochemical sensors,8 owing to their tunable electrical conductivity and surface properties.9 The intrinsic low thermal conductivity of CPs (at least one order of magnitude lower than inorganic materials) due to nanostructured interfaces or grains is one of the potential aspects in exploring CPs as thermoelectric materials.2,10–14Several CPs such as polyaniline (PANI), polypyrrole (PPY), polyphenylvinylene (PPV), polycarbazole (PC), and polyalkylthiophene have been tested for thermoelectric properties; they have low electrical conductivity and hence their potential as thermoelectric materials is limited.10 In order to enhance the thermoelectric properties of CPs, a wide variety of conducting fillers such as graphene, CNTs and composites with semiconducting inorganic materials have been introduced into the polymer matrix.15 Poly(3,4-ethylenedioxythiophene), popularly known as PEDOT, is a good choice as a polymer matrix due to its high environmental stability and electrical conductivity, in its oxidized state.16,17 PEDOT:PSS is a complex of PEDOT and poly(4-styrenesulfonate), in which the hydrophilic PSS component acts as a soluble matrix for the PEDOT domain, while also introducing a counter ion effect which helps to maintain the electrical neutrality of the complex. Thin films obtained from the aqueous dispersion of PEDOT:PSS consist of conducting PEDOT grains surrounded by insulating PSS shells.17 Pristine PEDOT:PSS films tend to exhibit low chain alignment with the presence of excess PSS.1–3 This kind of morphological feature results in a lower electrical conductivity of 1–10 S cm−1. Typical enhancement in the conductivity upon treatment has been explained through the removal of PSS components while forming extended conducting grains of PEDOT.17 There have been efforts to control the doping level of PEDOT chemically and electrochemically to optimize its thermoelectric performance. Treatment with organic solvents such as DMSO and ethylene glycol has been shown to enhance the electrical conductivity of PEDOT:PSS films.16 Blends of PEDOT:PSS with other organic and inorganic compounds have resulted in enhanced thermoelectric response.18–21 The effect of humidity on the thermoelectric performance of PEDOT:PSS has been studied wherein an apparent increase in the Seebeck coefficient was observed, and has been attributed to the morphological change after water absorption or the electrochemical reaction of PEDOT in air.22 It has been demonstrated that the Seebeck coefficient of PEDOT can be enhanced by chemical reduction23 and exposure to a reducing environment.24 Post-deposition treatment of PEDOT:PSS films with acids such as H2SO4 has been demonstrated to be effective in enhancing electrical conductivity.25,26 Recently, Mengistie et al., have investigated the thermoelectric performance of PEDOT:PSS paper by enhancing the electrical conductivity via post treatment with organic solvents and formic acid.27 Also, the available literature on this important class of developing thermoelectric material is limited to studies at room temperature. To the best of our knowledge, there has been only one previous report on the high temperature thermoelectric properties of PEDOT based films28 and none on PEDOT:PSS film. Hence, it is worthwhile to investigate the high temperature properties (but not exceeding 500 K) of PEDOT:PSS based thermoelectric films with the addition of methanol, followed by acid treatment, to explore both the effectiveness of these materials at elevated temperature and the highest temperature of stable operation; both should help expand the scope of polymer thermoelectrics. With this view, here we report the thermoelectric properties of PEDOT:PSS films in a post-deposition acid treatment process, in the temperature range 300–520 K, to explore the potential of this important polymer as a viable thermoelectric material at room temperature and high temperatures.
2. Experimental
In the present work, chemicals were used as received without further purification. PEDOT:PSS dispersion (PH1000, Clevios™) in water was employed as a conducting polymer solution. Films formed directly from the as received solution are denoted as ‘pristine’. 30 vol% of methanol was added to the water dispersion of PEDOT:PSS, in order to increase the wettability over the surface of the glass substrate. The quality of the PEDOT:PSS films seems much better after adding methanol when compared to the pristine water dispersion. Thick films (of thickness ∼3 μm) of PEDOT:PSS were obtained by drop casting 10 μL of the solution over cleaned 1 × 1 cm square glass substrates (Fischer Scientific), followed by drying on a hot plate at 120 °C for 15 minutes. Films thus formed are denoted as ‘untreated’. The films were then subjected to a surface treatment with 1 M H2SO4 (Sigma-Aldrich), at 140 °C for different durations (15 minutes, 2 and 4 h) and then washed with DI water followed by drying in a fume hood at room temperature, and are denoted as ‘acid-treated’. Surface morphology, microstructure and cross-sectional images were obtained using scanning electron microscopy (SEM) (Nova Nano 630 instrument, FEI Co., The Netherlands). Energy-dispersive spectroscopy (EDS) analysis was performed using an EDAX Genesis instrument (Mahwah, NJ) attached to the SEM column. The thickness of the films was measured using a Veeco Dektak 150 surface profilometer. The Raman spectra were recorded using a micro-Raman spectrometer (LabRAM ARAMIS, Horiba-Jobin Yvon), with notch filters that cut at 100 cm−1 and using a cobalt laser (473 nm, 5 mW at source) with a laser spot size of 1.5 μm. AFM imaging (MFP-3D, Asylum Research) was done using Si probes (model, RTESPA, spring constant 40 N m−1) in tapping mode and images were processed using WSXM software.29 X-ray diffraction (XRD) was used to investigate the evolution of the PEDOT fibrous morphology upon acid treatment (D8 Advance System from Bruker Corporation, equipped with a Cu Kα X-ray source, λ = 0.15406 nm). Electrical conductivity and the Seebeck coefficient of the films were measured in the temperature range 300–520 K, respectively employing the four probe and differential methods, using a commercial tester (RZ2001i, Ozawa Sciences). The electrical conductivity was measured first, at any temperature, followed by the Seebeck coefficient, by introducing a temperature gradient (0–10 K), using which, periodic test measurements with Ni foils always yielded data matching excellently with those reported by Burkov et al.30 The properties at higher temperatures were measured by uniformly heating a film placed in a furnace.3. Results and discussion
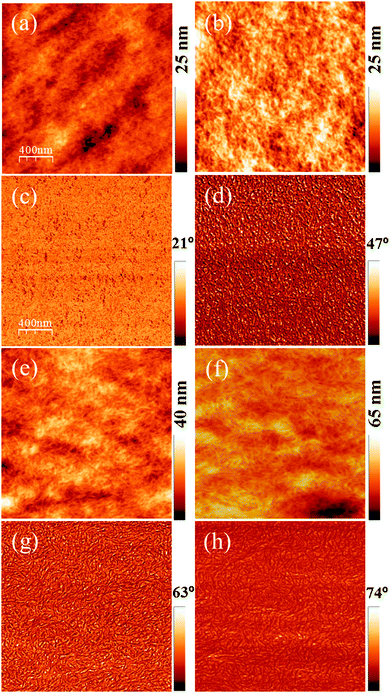
AFM images of untreated and acid-treated PEDOT:PSS films are shown in Fig. 1. Untreated PEDOT:PSS films were smooth, with a typical rms roughness of 2.4 nm as shown in Fig. 1a. Significant changes in the AFM topography were observed after acid bath treatment for 15 minutes as shown in Fig. 1b. Acid treatment is expected to remove the PSS domains, causing morphological changes. An increased rms roughness up to 3.5 nm and growth of small fibrous structures is clearly evident from the topography and phase images. Furthermore, extended bath treatment times of 2 and 4 h resulted in even more increased roughness of the films up to 4.4 nm as shown in Fig. 1e and f. The corresponding phase images (Fig. 1c, d, g and h) also reveal the extended formation of fibrous structures after acid treatment, as confirmed through XRD analysis and shown in the ESI,† Fig. S1. It is clearly evident that the acid treated film has a strong reflective peak at 2θ = 6.2°, indicating the ordering or evolution of fibrous structures of PEDOT upon acid treatment while the pristine PEDOT:PSS films exhibited an amorphous structure. Furthermore, the evolution of the second order peak at 13.1° is a clear indication of possible lamella stacking between the two distinct alternating orderings of PEDOT and PSS. These kinds of observations are in coherence with other literature reports.31–33Upon acid treatment, some of the PSS− ions get neutralized by the protons of H2SO4, causing the disappearance of Coulombic attraction between PEDOT and PSSH as shown in the below equations.
| H2SO4 → H+ + HSO4− pkal = −6.4 | (1) |
| PSSH → PSS− + H+ pka = −2.8 | (2) |
Since pkal of H2SO4 is higher than pka of PSSH, PSS− units get protonated upon treatment with sulphuric acid. Hence, the overall reaction can be expressed as below:
| H2SO4 + PSS− → HSO4− + PSSH | (3) |
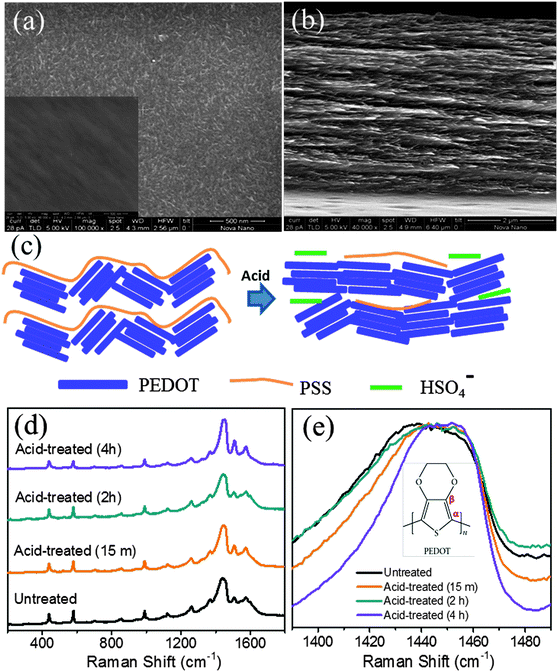
Fig. 2a and b show respectively the top and cross-sectional SEM images of the PEDOT:PSS films that has undergone acid treatment. Compared to the inset of Fig. 2a, which represents the top view SEM image of the untreated film, the emergence of a fibrous structure upon acid treatment is evident in Fig. 2a. The surface morphology of the films before acid treatment is shown in the inset of Fig. 2a. The cross sectional image shows that the fibrous nature is not limited to the surface alone and the film appears to be porous (see Fig. 2b). Possible conformational changes as a result of the removal of the PSS component are shown schematically in Fig. 2c. The Raman spectra for various PEDOT:PSS samples after acid treatment are compared with those of the untreated film as shown in Fig. 2d. Since PSS is a weak Raman scatterer, all peaks are assigned to the various normal modes of the vibration of atoms present in the PEDOT unit. The bands at higher wavenumbers such as 1573, 1503 cm−1 could be assigned to asymmetric stretching of Cα![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Cβ while the most intense peak at around 1440 cm−1 relates to symmetric stretching of Cα
Cβ while the most intense peak at around 1440 cm−1 relates to symmetric stretching of Cα![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Cβ of the five-membered thiophene ring. The peak at 1367 cm−1 corresponds to Cβ–Cβ inter-ring stretching, 1264 cm−1 represents Cα–Cα inter-ring stretching, 1105 cm−1 is due to C–O–C deformation, 988 cm−1 represents the C–C anti-symmetrical stretching mode, 702 cm−1 corresponds to symmetric C–S–C deformation, 573 cm−1 due to oxy-ethylene ring deformation and 441 cm−1 correspond to SO2 bending, confirms the doping of sulfate and bisulfate anions (from sulphuric acid) in PEDOT. The reference spectrum has a peak at 441 cm−1 which can be ascribed to the doping of PEDOT by the SO3− ion from PSS units. It should be noted that even for the untreated PEDOT:PSS films, doping of sulfite groups is caused from the PSS units. Hence, we do observe the 441 cm−1 band in either of the samples (untreated as well as acid treated PEDOT:PSS films). The shift in the position of C
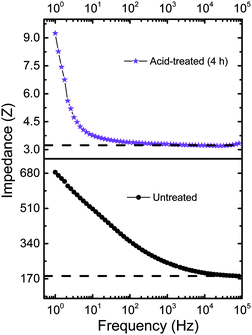
Cβ of the five-membered thiophene ring. The peak at 1367 cm−1 corresponds to Cβ–Cβ inter-ring stretching, 1264 cm−1 represents Cα–Cα inter-ring stretching, 1105 cm−1 is due to C–O–C deformation, 988 cm−1 represents the C–C anti-symmetrical stretching mode, 702 cm−1 corresponds to symmetric C–S–C deformation, 573 cm−1 due to oxy-ethylene ring deformation and 441 cm−1 correspond to SO2 bending, confirms the doping of sulfate and bisulfate anions (from sulphuric acid) in PEDOT. The reference spectrum has a peak at 441 cm−1 which can be ascribed to the doping of PEDOT by the SO3− ion from PSS units. It should be noted that even for the untreated PEDOT:PSS films, doping of sulfite groups is caused from the PSS units. Hence, we do observe the 441 cm−1 band in either of the samples (untreated as well as acid treated PEDOT:PSS films). The shift in the position of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (1438 cm−1) towards higher wavenumbers (see Fig. 2e) upon acid treatment is due to increased doping of PEDOT:PSS by bisulfate anions. The untreated PEDOT:PSS film shows the stretching vibration at 1438 cm−1, which gets shifted to 1451.15 cm−1 for a 4 h bath treated film. Several groups have also observed a similar shift, attributed to the doping of PEDOT by either a chemical or electrochemical manner.34,35 This conducting mechanism is associated with an increase of doping upon acid treatment that can be explained as follows. In the case of untreated PEDOT:PSS films, the amount of doping can be caused by the uni-negatively charged ions SO3− (from PSS segments) to create polarons or sometimes bipolaron carriers (maybe caused by excess HSO4− compared to PSS−) in the PEDOT backbone. Thus, the acid treatment helps to increase the doping level in PEDOT and hence enhanced electrical conductivity.35 A 50-fold decrease in impedance is observed in the acid-treated films as compared to the films untreated with acid, as in the Bode plots shown in Fig. 3. The decrease in impedance is concluded to be a direct consequence of improved electrical conductivity, supporting the hypothesis of removal of PSS components from the acid-treated films.
C (1438 cm−1) towards higher wavenumbers (see Fig. 2e) upon acid treatment is due to increased doping of PEDOT:PSS by bisulfate anions. The untreated PEDOT:PSS film shows the stretching vibration at 1438 cm−1, which gets shifted to 1451.15 cm−1 for a 4 h bath treated film. Several groups have also observed a similar shift, attributed to the doping of PEDOT by either a chemical or electrochemical manner.34,35 This conducting mechanism is associated with an increase of doping upon acid treatment that can be explained as follows. In the case of untreated PEDOT:PSS films, the amount of doping can be caused by the uni-negatively charged ions SO3− (from PSS segments) to create polarons or sometimes bipolaron carriers (maybe caused by excess HSO4− compared to PSS−) in the PEDOT backbone. Thus, the acid treatment helps to increase the doping level in PEDOT and hence enhanced electrical conductivity.35 A 50-fold decrease in impedance is observed in the acid-treated films as compared to the films untreated with acid, as in the Bode plots shown in Fig. 3. The decrease in impedance is concluded to be a direct consequence of improved electrical conductivity, supporting the hypothesis of removal of PSS components from the acid-treated films.
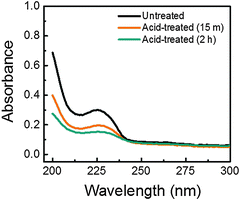
In order to confirm the removal of PSS from the films upon acid treatment, a few of the films were also subjected to UV-vis spectroscopy analysis, the result of which is shown in Fig. 4. It is seen that the absorbance shoulder peak at around 225 nm and the overall absorbance of the films reduced, with increase in acid treatment duration, as compared to the untreated film. The characteristic absorbance shoulder peak arises from the aromatic ring of the PSS components36 and the removal of PSS results in the enhanced transparency of the films, as reported elsewhere.37
The temperature dependence of the electrical conductivity of the films is shown in Fig. 5a. All the films show a metal-like behavior, with electrical conductivity decreasing with the increase in temperature, indicating that the carrier density in the films is high. Metal-like conduction is believed to be the dominant conduction mechanism in the temperature range of interest. Broadly, for lightly or moderately doped conjugated polymers, variable-range hopping dominates the conduction in the semiconductor regime of the semiconductor–metal transition, once localized states are formed in the band gap. It has been established38 that the inherent low dimensional nature of the constituent molecules of conjugated polymers such as PEDOT:PSS, is not a hindrance in achieving metal-like conductivity in these systems, since the electronic structure of such polymers can indeed resemble that of a disordered metal. The overlap of wave functions can occur in heavily doped conducting polymers. The effect of acid treatment is strikingly evident, from the enhancement in electrical conductivity as a result of the removal of PSS components in the films. Once PSS is slowly removed from the films, the conductivity is expected to be enhanced by (i) elongation and alignment of PEDOT chains and (ii) the reduction in density of the amorphous PSS regions. In such a series heterogeneous model of a conducting polymer with higher (PEDOT) and lower (PSS) conductivity constituents connected electrically in series, the total resistivity can be written as39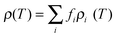 , where fi is the geometric factor given by
, where fi is the geometric factor given by 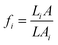 , L with A being the total effective length and cross-sectional area of the sample while Li and Ai are the length and cross-sectional area of a constituent material i with intrinsic resistivity ρi(T). In such a heterogeneous model, with increase in concentration of the conducting constituent as opposed to the non-conducting constituent, the overall conductivity becomes
, L with A being the total effective length and cross-sectional area of the sample while Li and Ai are the length and cross-sectional area of a constituent material i with intrinsic resistivity ρi(T). In such a heterogeneous model, with increase in concentration of the conducting constituent as opposed to the non-conducting constituent, the overall conductivity becomes
| σ(T) = 1/ρ(T) ≈ σ1(T)/f1 | (4) |
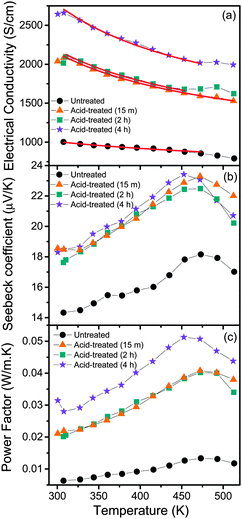 | ||
| Fig. 5 Temperature dependence of (a) electrical conductivity, (b) Seebeck coefficient and (c) power factor of untreated and acid-treated PEDOT:PSS films. Red lines represent the fitted curves, using eqn (2). | ||
Another important consequence of considering a series heterogeneous model as described above is the possible metal-like conductivity at high temperatures, similar to what is observed in other polymers such as polyacetylene and polyaniline.39 For extended metallic regions with intermediate insulating boundaries, the total conductivity which is due to a combination of quasi 1-D metallic conductivity and a temperature assisted tunneling process, where the former decreases and the latter increases with temperature, can be expressed as39
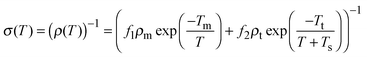 | (5) |
| Film | f 1 | f 2 | ρ m (×10−3 Ω cm) | ρ t (×10−4 Ω cm) | T m (K) | T t (K) | T s (K) |
|---|---|---|---|---|---|---|---|
| Untreated | 0.55 | 0.45 | 2.02 | 1.12 | 300 | 50 | 20 |
| Acid-treated (15 m) | 0.90 | 0.10 | 1.13 | 8.01 | 300 | 50 | 20 |
| Acid-treated (2 h) | 0.91 | 0.09 | 1.10 | 7.40 | 290 | 50 | 20 |
| Acid-treated (4 h) | 0.93 | 0.07 | 0.92 | 4.45 | 290 | 50 | 20 |
The Seebeck coefficient and the power factor of the films as a function of temperature are shown in Fig. 5b and c respectively (see Fig. S2, ESI†). The Seebeck coefficient of the films increases almost linearly with temperature up to around 460 K. Such a linear temperature dependence further supports the metal-like nature of electronic transport in PEDOT:PSS and the ideal metallic diffusion thermopower, for conduction involving states close to the Fermi level, may be expressed as40
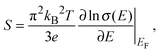 | (6) |
It is seen that acid treatment enhances not only the electrical conductivity of the films, but the Seebeck coefficient as well, which in turn results in improvement of the power factor. The simultaneous increase in electrical conductivity and the Seebeck coefficient is interesting, since in conventional thermoelectric materials, the two properties are often inversely related. From our studies, it is evident that acid treatment results in the removal of PSS and hence it should be concluded that PSS acts as a barrier for the transport of holes through the polymer network. The extended conjugated PEDOT chains, which form after the removal of PSS, act as a high mobility path for the transport of electrons. The result is an enhancement in electrical conductivity. The removal of PSS may also alter the local band structure of PEDOT:PSS. It is evident that such a possible change in the band structure may be responsible for the observed enhancement in the Seebeck coefficient. The film exposed to acid treatment for 4 h shows a 5-fold increase in the power factor at 460 K, compared to the untreated film. The huge surge in power factor upon acid treatment is both significant and encouraging, for further advancement of organic thermoelectrics. Beyond 460 K, it is observed that the Seebeck coefficient starts to decrease. A close observation of the electrical conductivity of the films shows a change in the slope of the conductivity curves around this temperature. Also, when films heated beyond 460 K are cooled, the electrical conductivity showed a hysteresis (not shown), while no such hysteresis was observed for films heated only up to 460 K. Hence, it is clear that beyond 460 K, there are irreversible changes in the film and the highest temperature of stable operation is around 460 K.
4. Conclusions
In conclusion, it has been shown that acid treatment of PEDOT:PSS thin films induces a simultaneous increase in electrical conductivity and the Seebeck coefficient. The thermoelectric power factor of the acid-treated films increases with temperature up to 460 K, with a peak power factor of 0.052 W m−1 K−1. This value is five times higher than the corresponding value of untreated PEDOT:PSS films. The enhancement is attributed to the loss of PSS from the PEDOT:PSS films besides the morphology change in the PEDOT grains, which enhances hole transport and is believed to modify the local band structure of the material, simultaneously increasing the Seebeck coefficient.Acknowledgements
The authors acknowledge the financial support provided by King Abdullah University of Science and Technology (KAUST), Saudi Arabia for carrying out this work.References
- O. Inganas, Nat. Photonics, 2011, 5(4), 201–202 CrossRef.
- Q. Zhang, Y. Sun, W. Xu and D. Zhu, Adv. Mater., 2014, 26(40), 6829–6851 CrossRef CAS PubMed.
- G. H. Kim, L. Shao, K. Zhang and K. P. Pipe, Nat. Mater., 2013, 12(8), 719–723 CrossRef CAS PubMed.
- A.-D. Bendrea, L. Cianga and I. Cianga, J. Biomater. Appl., 2011, 26(1), 3–84 CrossRef CAS PubMed.
- A. B. Kaiser, Adv. Mater., 2001, 13(12–13), 927–941 CrossRef CAS.
- P.-C. Wang, L.-H. Liu, D. Alemu Mengistie, K.-H. Li, B.-J. Wen, T.-S. Liu and C.-W. Chu, Displays, 2013, 34(4), 301–314 CrossRef CAS.
- R. A. Green, S. Baek, L. A. Poole-Warren and P. J. Martens, Sci. Technol. Adv. Mater., 2010, 11(1), 014107 CrossRef.
- A. Ramanavičius, A. Ramanavičienė and A. Malinauskas, Electrochim. Acta, 2006, 51(27), 6025–6037 CrossRef.
- G. A. Snook, P. Kao and A. S. Best, J. Power Sources, 2011, 196(1), 1–12 CrossRef CAS.
- N. Dubey and M. Leclerc, J. Polym. Sci., Part B: Polym. Phys., 2011, 49(7), 467–475 CrossRef CAS.
- M. Leijnse, K. Flensberg and T. Bjørnholm, Organic Optoelectronics, Wiley-VCH Verlag GmbH & Co. KGaA, 2013, pp. 467–486 Search PubMed.
- X. Gao, K. Uehara, D. D. Klug, S. Patchkovskii, J. S. Tse and T. M. Tritt, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72(12), 125202 CrossRef.
- M. He, F. Qiu and Z. Lin, Energy Environ. Sci., 2013, 6(5), 1352–1361 Search PubMed.
- O. Bubnova and X. Crispin, Energy Environ. Sci., 2012, 5(11), 9345–9362 CAS.
- Y. Du, S. Z. Shen, K. Cai and P. S. Casey, Prog. Polym. Sci., 2012, 37(6), 820–841 CrossRef CAS.
- J. Luo, D. Billep, T. Waechtler, T. Otto, M. Toader, O. Gordan, E. Sheremet, J. Martin, M. Hietschold, D. R. T. Zahn and T. Gessner, J. Mater. Chem. A, 2013, 1(26), 7576–7583 CAS.
- O. Bubnova, Z. U. Khan, A. Malti, S. Braun, M. Fahlman, M. Berggren and X. Crispin, Nat. Mater., 2011, 10(6), 429–433 CrossRef CAS PubMed.
- T.-C. Tsai, H.-C. Chang, C.-H. Chen and W.-T. Whang, Org. Electron., 2011, 12(12), 2159–2164 CrossRef CAS.
- A. Kim and S. Greg, Polymer Composites for Energy Harvesting, Conversion, and Storage, American Chemical Society, 2014, vol. 1161, pp. 147–163 Search PubMed.
- S. K. Yee, N. E. Coates, A. Majumdar, J. J. Urban and R. A. Segalman, Phys. Chem. Chem. Phys., 2013, 15(11), 4024–4032 RSC.
- B. Zhang, J. Sun, H. E. Katz, F. Fang and R. L. Opila, ACS Appl. Mater. Interfaces, 2010, 2(11), 3170–3178 CAS.
- W. Qingshuo, M. Masakazu, K. Kazuhiro, N. Yasuhisa and I. Takao, Appl. Phys. Express, 2014, 7(3), 031601 CrossRef.
- N. Massonnet, A. Carella, O. Jaudouin, P. Rannou, G. Laval, C. Celle and J.-P. Simonato, J. Mater. Chem. C, 2014, 2(7), 1278–1283 RSC.
- S. Liu, H. Deng, Y. Zhao, S. Ren and Q. Fu, RSC Adv., 2015, 5(3), 1910–1917 RSC.
- J. Ouyang, ACS Appl. Mater. Interfaces, 2013, 5(24), 13082–13088 CAS.
- Y. Xia, K. Sun and J. Ouyang, Adv. Mater., 2012, 24(18), 2436–2440 CrossRef CAS PubMed.
- D. A. Mengistie, C.-H. Chen, K. M. Boopathi, F. W. Pranoto, L.-J. Li and C.-W. Chu, ACS Appl. Mater. Interfaces, 2015, 7(1), 94–100 CAS.
- J. Wang, K. Cai and S. Shen, Org. Electron., 2014, 15(11), 3087–3095 CrossRef CAS.
- I. Horcas, R. Fernández, J. M. Gómez-Rodríguez, J. Colchero, J. Gómez-Herrero and A. M. Baro, Rev. Sci. Instrum., 2007, 78, 013105 CrossRef PubMed.
- A. T. Burkov, A. Heinrich, P. P. Konstantinov, T. Nakama and K. Yagasaki, Meas. Sci. Technol., 2001, 12, 264–272 CrossRef CAS.
- M. V. Fabretto, D. R. Evans, M. Mueller, K. Zuber, P. Hojati-Talemi, R. D. Short, G. G. Wallace and P. J. Murphy, Chem. Mater., 2012, 24, 3998 CrossRef CAS.
- M. A. Ali, H. Kim, C. Lee, H. Nam and J. Lee, Synth. Met., 2011, 161, 1347 CrossRef CAS.
- N. Kim, S. Kee, S. H. Lee, B. H. Lee, Y. H. Kahng, Y.-R. Jo, B.-J. Kim and K. Lee, Adv. Mater., 2014, 26, 2268 CrossRef CAS PubMed.
- W. W. Chiu, J. Travaš-Sejdić, R. P. Cooney and G. A. Bowmaker, Synth. Met., 2005, 155(1), 80–88 CrossRef CAS.
- M. Reyes-Reyes, I. Cruz-Cruz and R. López-Sandoval, J. Phys. Chem. C, 2010, 114(47), 20220–20224 CAS.
- A. K. Sarker, J. Kim, B.-H. Wee, H.-J. Song, Y. Lee, J.-D. Hong and C. Lee, RSC Adv., 2015, 5(64), 52019–52025 RSC.
- T. Koyama, T. Matsuno, Y. Yokoyama and H. Kishida, J. Mater. Chem. C, 2015, 3(32), 8307–8310 RSC.
- A. N. Aleshin, Phys. Solid State, 2010, 52(11), 2307–2332 CrossRef CAS.
- A. B. Kaiser, Rep. Prog. Phys., 2001, 64(1), 1 CrossRef CAS.
- N. Mott and E. Davis, Electronic processes in non-crystalline materials, Oxford, 1979 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5tc03145a |
| ‡ These authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2016 |