Open Access Article
Open Access ArticleThe influence of selected NSAIDs on volume phase transition in poly(2-(2-methoxyethoxy)ethyl methacrylate) hydrogels†
Magdalena N.
Olejniczak
*a,
Krzysztof
Piechocki
a,
Marcin
Kozanecki
a,
Kaloian
Koynov
b,
Agnieszka
Adamus
c and
Radosław A.
Wach
c
aDepartment of Molecular Physics, Faculty of Chemistry, Lodz University of Technology, Zeromskiego 116, 90-924, Lodz, Poland. E-mail: magdalena.olejniczak@p.lodz.pl
bMax Planck Institute for Polymer Research, Ackermannweg 10, D-55021 Mainz, Germany
cInstitute of Applied Radiation Chemistry, Lodz University of Technology, Wroblewskiego 15, 93-590 Lodz, Poland
First published on 26th January 2016
Abstract
Hydrogels exhibiting Volume Phase Transition (VPT) are considered as useful biomaterials for the preparation of various drug delivery systems. Such hydrogels are commonly based on thermo-responsive polymers, such as poly(2-(2-methoxyethoxy)ethyl methacrylate) (PMEO2MA), that have lower critical solution temperature (LCST) in aqueous solutions. In this work, PMEO2MA hydrogels were used as model systems to study the influence of encapsulated drugs, such as ibuprofen and salicylate sodium salts, on the temperature and dynamics of the VPT. Both thermo-optical analysis and differential scanning calorimetry have shown that the VPT becomes broader and shifts towards higher temperatures with increasing drug concentration. Three regimes of VPT in PMEO2MA gels were distinguished. The first two, related to the breaking of the strong water–polymer interactions and to the network collapse, slow down with increasing drug concentration. The last regime, corresponding to the slow diffusion of a residual solution from a collapsing network, becomes visible only for systems with high content of drug. Raman spectroscopy studies show that the observed effect is not connected to direct interactions between polymers and drugs. This suggests that the drug molecules are able to stabilise water–polymer interactions in thermo-responsive hydrogels. Consequently, they are able to modulate VPT and have a significant influence on the delivery process.
1. Introduction
High water content makes hydrogels biocompatible and results in a variety of biomedical applications, including contact lenses, soft implants and wound dressings.1 Recently, special interest was focused on smart drug delivery systems, such as micelles, vesicles, nanocapsules and nanospheres, dendritic polymers, bioconjugates and hydrogels. Thermo-responsive hydrogels exhibiting Volume Phase Transition (VPT)2 seem to be particularly attractive for this purpose because they enable precise control over the dose, release rate and delivery point of drugs.3 Increased temperature results in an imbalance between hydrophilic and hydrophobic polymer–water interactions, leading to the collapse of the polymer network and to the abrupt expulsion of liquid content (water or solution). Thus, reaching the VPT temperature (TVPT) may trigger the release of a drug that was incorporated into a polymer network. The transition temperature, which in turn controls drug release, depends on several factors that may be grouped into two main classes: (i) structure type factors: the ratio and the relative position of the hydrophilic and hydrophobic segments, network topology and tacticity and (ii) content type factors: solvent composition4 and the presence of additional solutes. For example, the extensively studied salting-out effect manifests itself in temperature dependent rapid gel collapse at a critical concentration of inorganic salt. It is thought that salts disrupt the hydration structure surrounding the polymer chains, causing a decrease in de-mixing temperature.5,6Additionally, aromatic compounds (for example, benzaldehydes) also cause lowering of the lower critical solution temperature (LCST) and TVPT in a linear polymer solution and gel, respectively. The observed effect depends on the molecular structure and concentration of added organic compounds. Although a clear decrease of LCST was found for all studied systems containing organic compounds, there was no correlation with either solubility or hydrophobicity of the aromatic molecules.7
In contrast to the inorganic salts and benzaldehydes, the anionic surfactant sodium dodecyl sulphate (SDS) brings about a large increase of TVPT or even a disappearance of VPT (salting-in effect). A similar but less pronounced effect was observed also in the case of cationic dodecyltrimethyl ammonium chloride (DTAC).8–10 Currently, there are two somewhat contradictory explanations of this effect. The first assumes that the surfactants absorb to the polymer chains and substantially disturb the hydrophilic/hydrophobic balance.9,10 The second one proposes a qualitative model for micelle formation inside the gel network.9
Investigations addressing the influence of biologically active agents on VPT in stimuli responsive hydrogels are very rare. One of the most essential studies in this field was published by Grinberg et al.11 The DSC study of polycationic and polyanionic poly(N-isopropylacrylamide) (PNIPAM) copolymer hydrogels showed that the presence of sodium ibuprofen and propranolol hydrochloride caused TVPT to have lower values in both cases. Additionally, Zuber et al. showed that the presence of bioactive molecules, such as lidocaine and its derivatives (hydrochloride and acetamide), had shifted the LCST of aqueous solutions of the linear copolymer of poly(2-(2-methoxyethoxy)ethyl methacrylate) and poly(N-vinylcaprolactam) to lower values.12 These data are intriguing in light of the opposite results obtained for PMEO2MA hydrogels in the present work.
Most of the aforementioned studies employed systems based on PNIPAM, which is considered to be the “gold standard”13 of thermo-responsive polymers, despite some recent suspicions of its monomer carcinogenicity.14 However, the knowledge of the impact of salt (the significant component of body fluids) and the ionic form of biologically active agents (used as drugs due to their good solubility in aqueous systems) on phase transition phenomena in PNIPAM alternatives is particularly important for future applications.
Hydrogels based on thermo-responsive polymers belonging to the poly(oligo(ethylene glycol)methacrylate) (POEGMA) group, such as PMEO2MA, are distinguishable by the tuneable, fully reversible and sharp VPT with small hysteresis.13 A multitude of synthesis methods useful for their preparation (free radical polymerisation – FRP,15 radiation-induced free radical polymerisation – RI-FRP,16 atom transfer radical polymerisation – ATRP,17 reversible addition-fragmentation chain transfer – RAFT18) and a variety of feasible structures (bare and decorated networks, brushes,19 stars,20 nano-, micro- and macrogels21) are also important advantages of POEGMA materials.
Importantly, the monomer of PMEO2MA appears to be non-toxic22 and non-immunogenic;23 hence, PMEO2MA hydrogels constitute promising materials for drug delivery applications.
T VPT is a critical parameter determining the drug release process; therefore, knowledge of the impact of the drug presence on VPT is highly desirable from both cognitive and practical points of view. In this work, the influence of the presence of non-steroidal anti-inflammatory drugs (NSAIDs) like (S)-ibuprofen (IBUNa) and salicylate (SALNa) sodium salts on the TVPT of PMEO2MA hydrogels is analysed within the context of their concentration. Selected drugs are good candidates for model substances due to their relatively simple chemical structure and small size. It is also necessary to underline that simple inorganic salts could not be regarded as a model of bioactive substances (drugs, enzymes, etc.) due to the lack of hydrophobic parts in their chemical structure. Hydrophobic segments may significantly influence the supramolecular structure of water that hydrated them.24 The results of thermo-optical analysis (TOA) presented herein are useful to determine a so-called “cloud point”, which reflects VPT. These results, supported by differential scanning calorimetry (DSC), allow for the determination of both the temperature and dynamics of this transition. Raman spectroscopy is used to provide more information about the causes of the observed effect at the molecular level.
2. Materials and methods
2.1. Sample preparation
Simultaneous polymerisation and cross-linking of PMEO2MA was attained via electron-beam irradiation of a mixture of 2-(2-methoxyethoxy)ethyl methacrylate (MEO2MA), 2-hydroxyethylmethacrylate (HEMA) and ethylene glycol dimethacrylate (EGDMA) (molar ratios: 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).16 Such composition of substrates and an irradiation dose of 30 kGy were selected to obtain a firm gel with good mechanical properties and, simultaneously, with a high degree of swelling (DS). The irradiation process was carried out at room temperature in 2 ml glass ampoules using the linear accelerator ELU-6 (Eksma, Russia) with a dose rate of ca. 5 kGy min−1. After irradiation, gels were conditioned for 24 hours at 50 °C to avoid phase separation being a post-polymerisation effect in the utilized system, as we observed previously.16
1).16 Such composition of substrates and an irradiation dose of 30 kGy were selected to obtain a firm gel with good mechanical properties and, simultaneously, with a high degree of swelling (DS). The irradiation process was carried out at room temperature in 2 ml glass ampoules using the linear accelerator ELU-6 (Eksma, Russia) with a dose rate of ca. 5 kGy min−1. After irradiation, gels were conditioned for 24 hours at 50 °C to avoid phase separation being a post-polymerisation effect in the utilized system, as we observed previously.16
After synthesis, the gels were immersed and equilibrated in deionized water (Merck Millipore Water Purification System) at 7 °C to extract both sol fraction and unreacted substrates. Water was exchanged every two days until the equilibrium degree of swelling has been reached. Then, the gels were freeze-dried.
2.2. Drug loading
Dry samples with a mass of 0.1–0.2 g were immersed in aqueous solutions of drugs used in significant excess. In the case of (S)-ibuprofen sodium salt, the following initial concentrations were used: 5, 10, 20, 30, 41, 100, 140, 182 mM, while for salicylate sodium salt: 6.5, 10, 20, 41, 100, 203 mM. Gel swelled with 154 mM sodium chloride solution (Baxter, 0.9% NaCl solution for infusion) was used as a negative control. Gels were immersed for at least ten days. This time was sufficient to establish thermodynamic equilibrium in the system, as the comparison of the results obtained for gels immersed for 10, 20 and 30 days has not shown differences – see Fig. S1A in the ESI.†Taking into account the potentially different solubility of used drugs in water and in the gel phase, the appropriate partition coefficient was determined as25,26
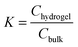 | (1) |
2.3. Gravimetric analysis
The degree of swelling at the equilibrium state (DSwatereq) in water, as well as in the drug solution (DSdrugeq), has been calculated as follows: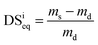 | (2) |
2.4. Thermo-optical analysis
Thermo-optical analysis (TOA) was performed in the bright field of an optical microscope. The TOA set consisted of a hot stage (Mettler FP 82), a control unit (Mettler FP 90) and a home-made cooling system (presented in Fig. S3 – see ESI†). In this method, the intensity of white light transmitted through the sample (I) is measured as a function of temperature, and analogous to nephelometry, the cloud point that accompanies VPT may be determined.27 Because TOA is a dynamic method (i.e., results are dependent on the scan rate), at first, the heating rate was optimised. Measurements were carried out for a neat PMEO2MA hydrogel at various heating rates (see Fig. S2 in the ESI†). The results acquired at heating rates that varied in the range of 0.5 to 2 °C min−1 are practically identical. Only the TOA curve recorded for faster heating (3 °C min−1) differed from the others. Thus, for further investigations, a 2 °C min−1 heating rate was chosen because it is close enough to thermodynamic equilibrium and simultaneously allowed for minimising the time of measurement.Raw data were normalized according to the formula:
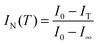 | (3) |
The experiments were carried out in triplicate and were fully repeatable (see Fig. S1B, ESI†). Presented cloud point temperatures are arithmetic averaged with standard deviation no greater than 0.8 °C.
2.5. Differential scanning calorimetry
Alternatively, measurements of VPT were conducted using a DSC technique. The DSC instrument (Q200 TA Instruments) was calibrated for both temperature and heat flow using, respectively, indium (melting temperature of 157 °C) and the synthetic sapphire standard with a well characterized specific heat capacity. Hydrogel samples with known mass in the range of 3 to 5 mg were sealed in hermetic aluminium pans and analysed in the temperature range between 0 °C and ca. 90 °C at a heating rate of 2 °C min−1 under a nitrogen flow of 50 ml min−1. Measurements were taken at least twice for all samples. Results were fully reproducible.2.6. Raman spectroscopy
The Raman spectra of drugs, drug solutions and hydrogels were recorded with the use of the FT Raman spectrometer MultiRAM (Bruker GmbH). All of the spectra were collected with a spectral resolution of 4 cm−1 and an excitation wavelength of 1064 nm (diode Nd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) YAG laser, 100 mW). During the measurements, hydrogel samples were placed into specially designed cells, guaranteeing a fixed position relative to the laser beam and protecting against water loss. To obtain the high quality spectra required, 32 scans were averaged. Because of the hydrogel thermo-responsivity, all operations and measurements were carried out in an air-conditioned room at 17 °C.
YAG laser, 100 mW). During the measurements, hydrogel samples were placed into specially designed cells, guaranteeing a fixed position relative to the laser beam and protecting against water loss. To obtain the high quality spectra required, 32 scans were averaged. Because of the hydrogel thermo-responsivity, all operations and measurements were carried out in an air-conditioned room at 17 °C.
3. Results and discussion
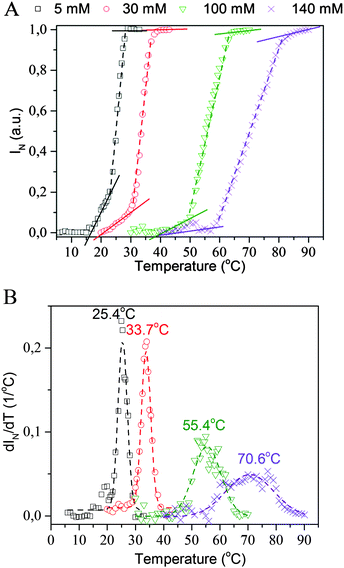
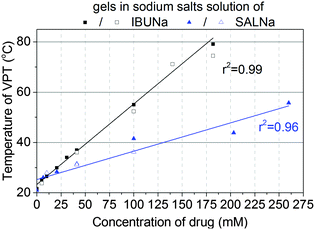
Fig. 1 presents selected TOA results obtained for PMEO2MA hydrogels immersed in aqueous solutions of IBUNa with various concentrations. It is clear that the “cloud point” (assumed to be represented by the deflection point of the TOA curve) shifts toward higher temperatures as the drug concentration increases. Surprisingly, the concentration dependence of the “cloud point” was found to be linear in a broad range of IBUNa content, up to 180 mM – as presented in Fig. 2. The TOA results are remarkably consistent with DSC data, as shown in Fig. 2, providing the TVPT directly. A similar tendency is also observed for another tested drug, i.e., SALNa, in contrast to a saline solution, which causes lowering of TVPT to 14 °C at a physiological concentration of NaCl (see Fig. S4 in the ESI†), as was expected based on literature reports.5,6 One can assume that this effect is correlated with a change in pH of sodium salt solutions. Alvarez-Lorenzo et al.28 found that TVPT slightly increased (from 32 to 34 °C) as pH decreased (from 8 to 3) in PNIPAM/chitosan interpenetrated networks. This effect was explained by the ionisation of the amino groups of chitosan chains in acidic medium. However, pH is an important factor in the case of polyelectrolytes, in which it is useless to explain the observed increase in TVPT for non-electrolyte PMEO2MA because PMEO2MA gels are pH-independent, as was previously reported by Paris.29 Moreover, performed tests showed that the pH only slightly varies (in the range of 7 to 8) in investigated PMEO2MA gels immersed in IBUNa solutions. Due to the same reason, the mechanism proposed by Tanaka et al. for poly(N-isopropylacrylamide-co-acrylic acid) should be rejected.30In non-ionic polymer networks such as PMEO2MA or poly(vinyl methyl methacrylate) (PVME), the polymer–polymer and water–polymer hydrophobic interactions, as well as the water–polymer and water–water H-bonds, play a key role.31
Thus, the most probable explanation is that the drug molecules interact inside the gel similar to surfactants. Two scenarios, which will be considered below, may be proposed in such a case:32
– drug molecules are adsorbed onto the polymer chain, directly disturbing the polymer–water interactions, and/or above critical micelle concentration (cmc) the drug molecules self-associate causing the polymer chains to coil to wrap the micelles,33
– drug molecules change the supramolecular structure of water (rearrangement of water–water H-bonds) and, as a result, this leads to different polymer chain stability in the system.
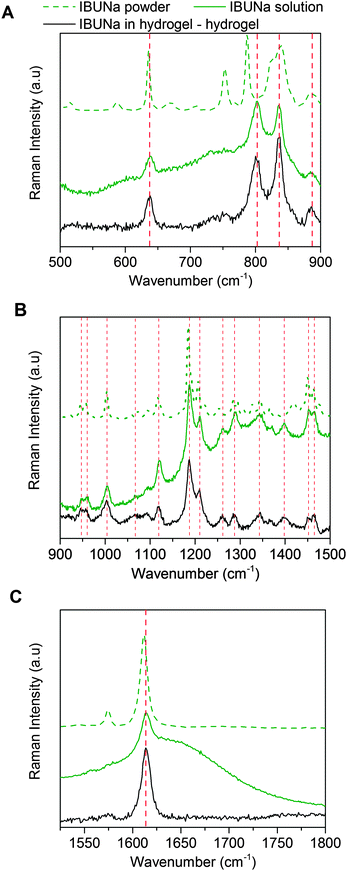
Raman spectroscopy is one of the most powerful methods used to test intermolecular interactions in water systems. In the case of hydrogels, it allows for determining the degree of chain hydration and its changes during various transitions, the water structure and even the activation energy of VPT.31,34,35Fig. 3 presents the Raman spectra of IBUNa powder and its aqueous solution in a fingerprint region, divided into three parts for clarity. The slight shift of particular bands toward higher wavenumbers results from hydration of the IBUNa molecules in dissolved form. To control the state of IBUNa in hydrogel, the differential Raman spectrum was calculated by extracting the spectrum of PMEO2MA hydrogel swollen in water from the spectrum of PMEO2MA hydrogel swollen in IBUNa solution. It was found that the positions of Raman peaks of IBUNa are exactly the same in the hydrogel and in the water solution. This means that there are no specific interactions between drug molecules and polymer chains. IBUNa molecules remain fully hydrated. Thus, the hypothesis that the IBUNa molecules are adsorbed onto the polymer network may be rejected.
The molecular structures of investigated drugs are similar to surfactant molecules with a hydrophobic part and a strongly hydrophilic ionic group. Due to these structural features, they are able to form micelles (cmc for IBUNa and SALNa are 180 mM33 and 705 mM,36 respectively). Nevertheless, the range of concentration investigated in this study does not exceed cmcs. Only one gel sample in 180 mM IBUNa solution reaches the cmc, but no change in TVPT dependence of drug concentration was observed. Taking into consideration the second scenario it is worth noticing that the polymer chains consist of hydrophilic (easily forming H-bonds) and hydrophobic segments which are encased by water cage-like structures. According to Tanford et al.,37 in such type of systems, water molecules may form highly ordered structures, called ice-bergs, in the vicinity of hydrophobic polymer chains. The ice-bergs, with water molecules arranged similarly to ice Ih, stabilise the polymer chains in a water environment. Taking into account the lack of direct interactions between the drug and the polymer, it is worth comparing drug concentrations in the gel phase with respect to the initial solution. The partition coefficient of IBUNa and SALNa for various samples was estimated. As the results presented in Table 1 show, the coefficient is almost independent of the initial drug concentration over the investigated concentration range, and the average values are 1.49 ± 0.04 and 0.99 ± 0.06 for IBUNa and SALNa, respectively. A similar tendency was found for another non-ionic PVME gel used as a Triton adsorbent.38 It is also important to note that the DSibueq to DSwatereq ratios are greater than unity. The results discussed herein are also consistent with the results obtained by Alvarez-Lorenzo et al.28 for different thermo-responsive polymer systems – PNIPAM/chitosan interpenetrated networks. This effect was explained by the fact that the free amino groups located along the chitosan chain are cationically charged over a wide range of physiological pHs.28 Lowe et al. found that hydrophobic ibuprofen (in the form of acid) induced a decrease of the PNIPAM-based microgels'' size below the LCST of PNIPAM and sharpened the VPT.12 It was also reported for PMEO2MA hydrogels29 that DSeq reached greater values if the gels were swelled in more basic solutions. Ibuprofen sodium salt, used in these studies, is the sodium salt of weak acid, which results in a slightly alkaline medium (pH varied between 7 and 8). Thus, the salting in effect was observed. Interestingly, ibuprofen acid incorporated into PNIPAM copolymer hydrogels brought about a drastic decrease of its DSeq (salting out effect).12 The constant partition coefficient, as well as DSibueq to DSwatereq ratios, was much greater than unity, which may suggest the formation of a supramolecular structure within the hydrogel, as the simple drug adsorption to the polymer network was excluded by Raman spectroscopy.
| Sodium salt of | C drugo (mM) | DSdrugeq/DSwatereq | C drughydrogel (mM) | C drugbulk (mM) | K |
|---|---|---|---|---|---|
| Ibuprofen | 5 | 1.23 | 6.76 | 4.63 | 1.46 |
| 10 | 1.89 | 12.76 | 8.51 | 1.50 | |
| 20 | 1.66 | 27.38 | 18.81 | 1.46 | |
| 30 | 1.45 | 43.71 | 28.00 | 1.56 | |
| 75 | 1.68 | 96.00 | 64.29 | 1.50 | |
| Salicylate | 6.5 | 0.82 | 6.34 | 6.73 | 0.94 |
| 10 | 0.89 | 10.08 | 10.92 | 0.92 | |
| 20 | 0.88 | 20.76 | 19.57 | 1.06 | |
| 100 | 0.87 | 102.12 | 97.92 | 1.04 | |
The other observed effect relates to the broadening of VPT with increasing drug concentration. This is clearly evident in Fig. 1B, where the temperature dependences of dIN/dT are presented. This broadening suggests that the VPT becomes continuous in the presence of drug molecules. According to Shibayama and Tanaka,39 the discrete or continuous character of the VPT transition relates directly to the temperature dependence of osmotic pressure in the gel. It is easy to imagine that the presence of an ionic substance (drug) in the gel strongly influences this dependence. However, its precise determination is difficult, taking into account all variables changeable during VPT, including the concentration of IBUNa inside the gel, gel volume and intermolecular interactions within the system.
Analysis of the TOA results reveals the presence of three regimes in the VPT. The slopes corresponding to each of them are plotted as a function of IBUNa concentration in Fig. 4 and shown, as straight lines, in Fig. 1A as well. The slopes related to the first and second regimes decrease with IBUNa content. This means that the VPT slows down if the drug concentration increases. The third regime is extremely narrow and practically invisible for solutions with a low concentration of IBUNa, while for higher drug concentrations (above 41 mM), it becomes visible.
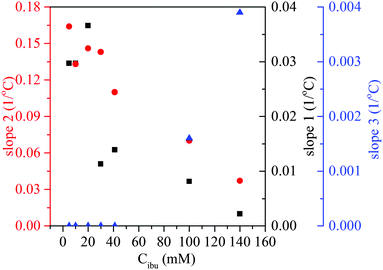 | ||
| Fig. 4 VPT dynamics in the presence of ibuprofen sodium salt. Dynamics of VPT transition represented by slopes of TOA dependences shown in Fig. 1 in three regimes: slope 1 – squares, slope 2 – dots, slope 3 – triangles (see text for details). | ||
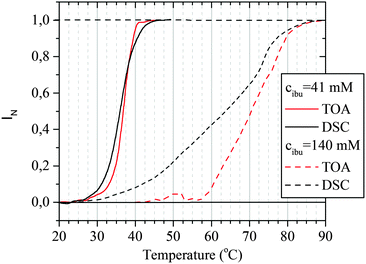
To explain these effects, the comparison of TOA and DSC results was done. Presented in Fig. 5, data for the hydrogel containing 41 mM of IBUNa are representative also for all systems with lower IBUNa content. DSC thermograms show that, although the VPT starts at the same temperature of ca. 25 °C independent of the drug concentration, the dynamics of the transition are completely different for low and high drug content. Transition is rapid and discontinuous for samples with low drug concentration. This means that most of the water–polymer interactions break rapidly in the beginning, which is manifested by the sharp energetic effect. Consequently, the hydrogel abruptly becomes thermodynamically instable. The polymer response is also very fast, and rapid collapse of the polymer network leads to an increase of opaqueness manifested by the “cloud point”. Higher drug concentration (≥41 mM) causes significant redistribution of intermolecular interactions. Strong water–polymer interactions break gradually and disappear completely at higher temperatures. Evidently, IBUNa stabilised the PMEO2MA–water system. Thus, the hydrogel collapses slowly upon approaching the TVPT (the first regime in TOA results). The higher the IBUNa content, the slower the process. If a particular number of water–polymer interactions decay, the network becomes unstable. As a result, the gel abruptly reduces its volume. This phase corresponds to the second regime visible in TOA curves and results in the “cloud point”. The mentioned herein effects may also be strengthened by the changes in IBUNa concentration inside the gel. It is very probable that the drug molecules, which are much bigger than the water molecules, diffuse outside the gel with a lower rate than the collapse of the gel. As a result, the IBUNa concentration systematically increases with progressing VPT. Thus, the VPT becomes broader for systems with higher drug content. Indeed, micro-Raman spectroscopy investigations performed recently for similar hydrogel systems (based on PVME) containing IBUNa showed that the drug concentration inside the gel may exceed the critical concentration during the VPT, and crystallisation of IBUNa was observed (see Fig. S5 in the ESI†). Additionally, the presence of the third regime in the TOA experiment, observed for concentrated systems (Fig. 1), supports this hypothesis. The residual amount of IBUNa with strongly limited diffusivity probably protects the gel against complete collapse. In this phase, the polymer network is partially relaxed and is unable to pull the liquid content out. Contrarily, if the initial drug concentration is low, the internal pressure induced by a rapidly collapsing network is high enough to pull out the whole liquid content. Thus, the third regime is not present for the systems with low IBUNa concentration.
4. Conclusions
The influence of the presence of some selected commonly used non-steroidal anti-inflammatory drugs (ibuprofen and salicylate sodium salts) on the VPT in PMEO2MA hydrogels synthesised via electron beam irradiation was the subject of the presented investigations. The results of thermo-optical analysis and differential scanning calorimetry showed that the VPT temperature depends on both drug concentration and its chemical structure. Increasing the content of ibuprofen and salicylate sodium salts resulted in a shift of TVPT towards higher temperatures. Moreover, the VPT became broader. Comparison of TOA and DSC results allowed for distinguishing three regimes of VPT in PMEO2MA gels. The first two, related to the breaking of the strong water–polymer interactions and to the network collapse, respectively, slow down with increasing drug concentration. The last regime, corresponding to the slow diffusion of a residual solution from a collapsing network, becomes visible only for systems with high drug content. Such behaviour suggests that the drug molecules stabilised the water–polymer interactions. Comparison of gravimetric and Raman spectroscopy results suggests that the observed effect is due to the stabilisation of water–polymer interactions or water–water structures.Further investigations are needed to explain the mechanism of the observed effect in detail, however the presented results are indicative of various applications (not only biological). The strong correlation between the TVPT and drug concentration was demonstrated for PMEO2MA but it is probably visible also for other polymer hydrogels. Based on the presented results it may be stated that it is necessary to build a suitable polymer carrier (with particular chemical structure and/or morphology) not only for a particular drug but also for a particular dose.
Acknowledgements
The authors are deeply grateful to Dr S. Kadlubowski and MSc M. Matusiak for delivery of the hydrogel samples. This work was financially supported by the following projects: 2013/09/B/ST4/03010 (Polish National Science Centre) and Young Scientists' Fund at the Faculty of Chemistry, Lodz University of Technology (No. W-3/FMN/24G/2015).References
- A. S. Hoffman, Adv. Drug Delivery Rev., 2012, 64, 18 CrossRef.
- J. Kopeček, Eur. J. Pharm. Sci., 2003, 20, 1 CrossRef.
- A. Amit, B. Ajazuddin, K. Junaid, S. Swarnlata and S. Shailendra, Eur. J. Pharm. Biopharm., 2014, 88, 575 CrossRef PubMed.
- K. Otake, H. Inomata, M. Konnoa and S. Saito, Macromolecules, 1990, 23, 283 CrossRef CAS.
- H. Inomata, S. Goto, K. Otake and S. Saito, Langmuir, 1992, 8, 687 CrossRef CAS.
- K. van Durme, H. Rahier and B. van Mele, Macromolecules, 2005, 38, 10155 CrossRef CAS.
- C. Hofmann and M. Schonhoff, Colloid Polym. Sci., 2009, 287(2009), 1369 CAS.
- C. Wu and S. Zhou, J. Polym. Sci., Part B: Polym. Phys., 1996, 34, 1597 CrossRef CAS.
- H. Inomata, S. Goto and S. Saito, Langmuir, 1992, 8, 1030 CrossRef CAS.
- D. Schmaljohann, Adv. Drug Delivery Rev., 2006, 58, 1655 CrossRef CAS PubMed.
- V. Y. Grinberg, T. V. Burova, N. V. Grinberg, A. S. Dubovik, A. Concheiro and C. Alvarez-Lorenzo, Langmuir, 2014, 30, 4165 CrossRef CAS PubMed.
- S. Zuber, K. Landfester, D. Crespy and A. Popa, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 3308 CrossRef CAS.
- J. F. Lutz, O. Akdemir and A. Hoth, J. Am. Chem. Soc., 2006, 128, 13046 CrossRef CAS PubMed.
- A. S. Wadajkar, B. Koppolu, M. Rahimi and K. T. Nguyen, J. Nanopart. Res., 2009, 11, 1375 CrossRef CAS.
- Y. Maeda, T. Kubota and H. Yamauchi, Langmuir, 2007, 23, 11259 CrossRef CAS PubMed.
- S. Kadlubowski, M. Matusiak, J. Jenczyk, M. Olejniczak, M. Kozanecki and L. Okrasa, Radiat. Phys. Chem., 2014, 100, 23 CrossRef CAS.
- J. A. Yoon, T. Kowalewski and K. Matyjaszewski, Macromolecules, 2011, 44, 2261 CrossRef CAS.
- A. Zengin, Y. Ertan and C. Tuncer, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 954 CrossRef CAS.
- S. I. Yamamoto, J. Pietrasik and K. Matyjaszewski, Macromolecules, 2007, 40, 9348 CrossRef CAS.
- K. Knop, D. Pretzel, A. Urbanek, T. Rudolph, D. H. Scharf, A. Schallon, M. Wagner, S. Schubert, M. Kiehntopf, A. A. Brakhage, F. H. Schacher and U. S. Schubert, Biomacromolecules, 2013, 14, 2536 CrossRef CAS PubMed.
- Z. Hu, T. Cai and C. Chi, Soft Matter, 2010, 6, 2115 RSC.
- H. Vihola, A. Laukkanen, L. Valtola, H. Tenhu and J. Hirnoven, Biomaterials, 2005, 26, 3055 CrossRef CAS PubMed.
- L. Tang, Y. Yang, T. Bai and W. Liu, Biomaterials, 2011, 32, 1943 CrossRef CAS PubMed.
- M. Bester-Rogac, Acta Chim. Slov., 2009, 56, 70 CAS.
- J. Tong and J. l. Anderson, Biophys. J., 1996, 70, 1505 CrossRef CAS PubMed.
- C. Kostmar, T. Sells, N. Taylor, D. E. Liu, J. M. Prausnitz and C. J. Radke, Macromolecules, 2012, 45, 9177 CrossRef.
- Y. C. Bae, S. M. Lambert, D. S. Soane and J. M. Prausnitz, Macromolecules, 1991, 24, 4403 CrossRef CAS.
- C. Alvarez-Lorenzo, A. Concheiro, A. S. Dubovik, N. V. Grinberg, T. V. Burova and V. Y. Grinberg, J. Controlled Release, 2005, 102, 629 CrossRef CAS PubMed.
- R. Paris and I. Quijada-Garrido, Eur. Polym. J., 2010, 46, 2156 CrossRef CAS.
- H. Shunsuke, Y. Hirokawa and T. Tanaka, J. Chem. Phys., 1987, 87, 1392 CrossRef.
- M. Pastorczak, G. Dominguez-Espinosa, L. Okrasa, M. Pyda, M. Kozanecki, S. Kadlubowski, J. M. Rosiak and J. Ulanski, Colloid Polym. Sci., 2014, 292, 1775 CAS.
- E. Kokufuta, Y.-Q. Zhang, T. Tanaka and A. Mamada, Macromolecules, 1993, 26, 1053 CrossRef CAS.
- R. Rodríguez, C. Alvarez-Lorenzo and A. Concheiro, Eur. J. Pharm. Sci., 2003, 20, 429 CrossRef.
- M. Pastorczak, M. Kozanecki and J. Ulanski, Polymer, 2009, 50, 4535 CrossRef CAS.
- G. E. Walrafen, M. S. Hokmabadi and W.-H. Yang, J. Phys. Chem., 1988, 92, 2433 CrossRef CAS.
- Y. Tanaka, Y. Miyazaki, S. Yakou and K. Takayama, Pharmazie, 2007, 62, 41 CAS.
- C. Tanford, The Hydrophobic Effect: Formation of Micelles and Biological Membranes, J. Wiley, 2nd edn, 1980 Search PubMed.
- K. Yamagiwa, M. Katoh, M. Yoshida, A. Ohkawa and H. Ichijo, Water Sci. Technol., 1997, 37, 213 CrossRef.
- M. Shibayama and T. Tanaka, Volume phase transition and related phenomena of polymer gels, Responsive gels: volume transitions I, Springer, Berlin, Heidelberg, 1993, vol. 109, p. 1 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5tb02217g |
| This journal is © The Royal Society of Chemistry 2016 |