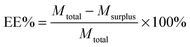Novel drug delivery nanosystems based on out-inside bifunctionalized mesoporous silica yolk–shell magnetic nanostars used as nanocarriers for curcumin†
Peilin
Huang‡
a,
Baozhen
Zeng‡
b,
Zhuoxian
Mai
a,
Juntao
Deng
a,
Yueping
Fang
a,
Wenhua
Huang
*b,
Hongwu
Zhang
*b,
Jinying
Yuan
c,
Yen
Wei
c and
Wuyi
Zhou
*a
aInstitute of Biomaterials, Department of Applied Chemistry, College of Materials and Energy, South China Agricultural University, Guangzhou, 510642, China. E-mail: zhouwuyi@scau.edu.cn
bDepartment of Anatomy, Guangdong Provincial Key Laboratory of Construction and Detection in Tissue Engineering, Southern Medical University, Guangzhou 510515, China. E-mail: zhanghwu@mail2.sysu.edu.cn; huangwenhua2009@139.com
cKey Lab of Organic Optoelectronics & Molecular Engineering of Ministry of Education, Department of Chemistry, Tsinghua University, Beijing, 100084, China
First published on 12th November 2015
Abstract
Mesoporous silica has aroused lots of interest in biomedical fields, in which novel kinds of mesoporous silica with tunable mesoporosity and size have attracted much attention, but functionalization or multi-functionalization inside the mesopores of these structures is still rarely detected. Among all the functionalized molecules, beta cyclodextrin hydrate (β-CD), a kind of hydrophilic non-toxic carrier for hydrophobic drugs, can increase the solubility and bioavailability of drugs, acting as a pH responsive “gate” when functionalized on the surface of mesoporous silica. Herein, we functionalized β-CD inside novel kinds of mesoporous silica or magnetic mesoporous silica as the physical binding sites for the model drug curcumin through an out-inside two step bifunctionalization process including a vacuum pumping recrystallization drug loading process. According to the physical characterization with XRD, FT-IR, XPS, solid state NMR, BET, TG, DTA, TEM and DLS, the drug delivery nanosystems were successfully fabricated and contained nano-formulations of curcumin. In vitro cell testing, including Cell Counting Kit-8, Hoechst 33342 staining, hemolysis experiments and cell apoptosis, indicated that through the bifunctionalization, the biocompatibility of the mesoporous silica with cells could be improved, and the toxicity of the drug delivery nano-formulations towards two kinds of cancer cells, SK-HEP1 and HepG2, was significantly increased compared with free curcumin. In vitro release studies revealed that the nano-formulations contained more suspended curcumin molecules as described by the Higuchi models and showed enhanced pH responsive release properties, and that the magnetic nano-formulations exhibited alternating magnetically controlled release properties. The confocal microscopy analysis results revealed the obviously increasing intercellular release and uptake of both nano-formulations of curcumin by SK-HEP1 cells and the “on” and “off” stages of the alternating magnetism responsive intercellular release properties of the fabricated magnetic nano-formulations. The bifunctionalization process combined with the nanostar structure has immense potential in the drug delivery field, especially for hydrophobic drugs.
Introduction
Mesoporous silica has aroused lots of interest in biomaterials fields, such as drug delivery nanosystems, tissue scaffolds, tumor and cancer diagnosis, etc. There are many reports concerning novel shapes of mesoporous silica and functionalized mesoporous silica with smart controlled release. Novel kinds of mesoporous silica with tunable mesoporosity and size, first reported by Zhang et al.,1 have aroused lots of attention among researchers in fields such as catalysis,2 biomaterials and drug delivery materials,3 adsorption and environmental protection,4 and bioengineering5. However, reports of functionalizaton or multi-functionalization inside the mesopores of these materials are still rare, by which these novel structures could be functionalized with more physical or chemical drug binding sites and respond to more environmental conditions. This would promote the drug loading properties inside the mesopores,6 which could be widely applied in more biological fields.7 However, large mesopores can be functionalized with specific sites, which can not only be combined with drug molecules but can also prolong the drug release period and enhance the drug release properties. Beta cyclodextrin hydrate (β-CD), a kind of hydrophilic non-toxic carrier for hydrophobic drugs, can protect vitamins and natural colours8 and increase drug suspension, as well as being soluble and exhibiting stable properties in water,9 which could be a great help in increasing the bioavailability of drugs and delivery in vivo. What's more, cyclodextrin and beta cyclodextrin hydrate have been used to block drug molecules inside mesoporous channels, acting as a pH responsive “gate” on the surface of mesoporous silica.10 However, the surface nested β-CD are influenced by many effects and might difficultly keep nested state when applied in vivo before these drug delivery nanosystems were distributed to the targeted organs. Worse still, surface β-cyclodextrin hydrate may limit the surface functionalization of other specific binding sites, which might hinder the grafting of other specific targeted or functionalized molecules on the surface.Curcumin, a kind of yellow hydrophobic pigment obtained from the rhizome of the herb Curcuma longa,11 exhibits lots of bioactivity, which could be applied in therapy for cancer or tumors, heart failure and other diseases.12 But curcumin molecules were shown to be unstable to a series of physical and chemical conditions, i.e., heat, ions, amino groups or alkali medium. Nano-formulations of curcumin have been previously prepared, such as silica aggregates,13 silica nanoparticle conjugates14 and silica xerogels,15 which aroused lots of interest and not only increased the delivery of the curcumin drugs, but also enhanced its stability and suspension properties. However, the controllable and targeted release of curcumin drugs is still neglected, leaving much to be desired in the delivery properties. In addition, curcumin molecules are sensitive to iron ions,16 as they are good chelators.17 When they are exposed to even a few Fe(III) ions or iron ions in Fe3O4 nanoparticles, the structure of the molecules might be affected in a manner that remains unclear. Mesoporous silica, with tunable structure and surface rich silanol groups, can connect drug molecules and inorganic nanoparticles, acting as a bridge. Hence, a novel structure of mesoporous silica, inside which the Fe3O4 nanoparticles can be encapsulated, is completely necessary. Using this novel structure to fabricate magnetic drug delivery nano-formulations could reduce the effect of ferric ions on curcumin molecules, and increase the controllable and targeted release properties of these drug delivery nano-formulations. Herein, we firstly functionalized β-CD inside the large mesopores of a novel nanostar structure with magnetic cores to act as the inside gate through out-inside two step functionalization technology.6 The surface functionalized β-CD could provide more physical binding sites for curcumin and enhance the interactions between the drug molecules and the inorganic materials,18 and was combined with a vacuum pumping recrystallization drug loading process,19 instead of using ammonia conjugated groups,20 alkaline materials,21 covalent bonding to drug molecules22 and heat treatment during the drug loading process. This might influence the molecular structure and stability of curcumin during the drug loading process and storage.
The results indicate that the inside-grafted β-CD molecules provide more physical binding sites for drug molecules with more branched chains on the benzene rings, and increase drug loading inside the meso-channels. Through physical characterizations, such as XRD, FTIR, XPS, solid state NMR, BET, TEM, DLS, etc., the out-inside stepwise functionalization process was confirmed and the fabrication of drug delivery nanocarriers for curcumin was monitored. In vitro cell viability tests, Hoechst 33342 staining, hemolysis tests and cell apoptosis tests revealed that the bifunctionalized nanostars exhibit improved biocompatibility with cells compared with single materials, and significantly increase the toxicity of curcumin to two kinds of cancer cells, i.e., SK-HEP1 cells and HepG2 cells, compared with free curcumin. The in vitro release profile revealed that loading curcumin on this mesoporous silica can increase the amount of curcumin in suspension as described by the Higuchi models, and that the increase in the number of functionalized sites can also improve the suspension properties of curcumin molecules. Meanwhile, the pH responsive release showed that the release rate significantly increases at lower pH values. In addition, the cellular uptake experiments revealed that the intercellular release and uptake of curcumin in SK-HEP1 cells were increased due to the novel delivery properties of the nano-formulations. The “on–off” alternating magnetic field (AMF) induced release indicated that the magnetic bifunctionalized nanostar drug delivery systems are responsive to magnetism during controlled drug release, and that the intercellular drug release increases when the alternating magnetic field is applied. The bifunctionalization process combined with the nanostar structure and magnetic cores has immense potential in the drug delivery field.
Materials and methods
Reagents and materials
Hexadecyltrimethylammonium toluene-p-sulphonate (CTAT, 98%), (3-isocyanatopropyl)-triethoxysilane (IPTS, 95%), tris(hydroxymethyl)aminomethane (AHMPD, AR), (3-mercaptopropyl)trimethoxysilane (KH590, 95%), and β-cyclodextrin (β-CD, AR) were supplied by Sigma-Aldrich. Tetraethylorthosilicate (TEOs) and xylene were analytical reagent grade and were purchased from Guangzhou Chemical Reagent Factory, China. All the other reagents were supplied by Sigma-Aldrich unless otherwise noted.Fabrication of nanostars and magnetic nanostars
Nanostar shaped mesoporous silica was fabricated through a soft-templating process as reported,1 with minor adjustments. To a reactor, 1.50 g of CTAT, 0.11 g of AHMPD and 50 mL of deionized water were added. The mixed solution was mechanically stirred at 1200 rpm in a 353 K water bath for 1 h. After that, 7.8 mL of TEOs was quickly injected into the reactor and reacted at 353 K for the next 2 h. The as-synthesised nanostars were collected through a filtration process and dried at 353 K for further use. Magnetic nanostars were fabricated through an electrostatic process combined with a soft-templating process, which is shown in Fig. 1(a). To a reactor, 0.11 g of AHMPD, a certain amount of a water-based ferrofluid (0.025 g mL−1 Fe3O4/water, as prepared in our previous work,23 detailed steps are shown in the ESI†) and 50 mL deionized water were added. The mixed solution was vigorously stirred at 1200 rpm at 333 K for 10 min to settle the solution. After a while, 2 mL of TEOs was added dropwise to the reactor, and the mixed solution was left to react for 50 min. After the core forming reaction, 1.50 g of CTAT was added into the reactor, and the temperature was altered to 353 K to provide the soft-templating conditions for 1 h. Then, 5.8 mL of TEOs was injected quickly into the reactor, and the mixed suspension was stirred at 1200 rpm at 353 K for another 2 h. Finally, the as-synthesised magnetic nanostars were collected using a magnetic separation and filtration process, and dried at 353 K for further use. In this way, we synthesised three types of magnetic nanostars (MNS), namely M2NS, M1.5NS and M1NS, by adding different amounts of water-based ferrofluid, i.e., 2 mL, 1.5 mL and 1 mL. The three samples contained different amounts of Fe3O4 nanoparticles: 0.05 g, 0.0375 g and 0.025 g, equal to 2.34%, 1.75% and 1.17% Fe3O4/SiO2, respectively.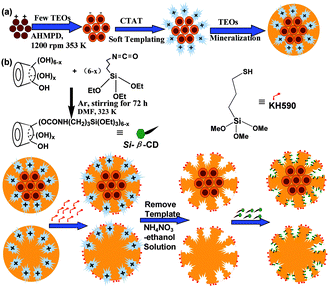 | ||
| Fig. 1 Schemes of the fabrication of magnetic nanostars with template (a) and the bifunctionalized nanostar shaped drug delivery systems (b). | ||
Fabrication of bifunctionalized nanostars and magnetic nanostars
Bifunctionalized nanostars and magnetic nanostars were synthesised through out-inside bifunctionalization technology. Firstly, 8 g of dried β-CD was reacted with 10 mL of (3-isocyanatopropyl)triethoxysilane (IPTS) with N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in 50 mL of N,N-dimethylformamide (DMF) at 323 K with magnetic stirring under an Ar atmosphere for 72 h to form Si–β-CD,24 as shown in Fig. 1(b); the obtained solution was stored at 278 K under dry conditions for further use. 1.45 g of dried nanostars or magnetic nanostars with template (containing 1.0 g of pure SiO2) were added into a reactor with 100 mL xylene and 10 mmol of KH590 (thiol groups). The mixture was refluxed for 24 h at 363 K under a N2 atmosphere, and the powder was collected through a filtration process and washed three times with xylene and ethanol. To achieve the functionalization of the inside pores, the samples were further refluxed in 100 mL xylene with the same volume of DMF solutions containing 0.60 mmol Si–β-CD, 0.45 mmol Si–β-CD, 0.30 mmol Si–β-CD, 0.15 mmol Si–β-CD, 0.10 mmol Si–β-CD and 0.05 mmol Si–β-CD at 363 K for 24 h under a N2 atmosphere, to form different bifunctionalized nanostars, i.e., F0.60NS, F0.45NS, F0.30NS, F0.15NS, F0.10NS and F0.05NS, and magnetic nanostars, i.e., F0.60M2NS, F0.45M2NS, F0.30M2NS, F0.15M2NS, F0.10M2NS, F0.05M2NS, F0.60M1.5NS and F0.60M1NS. These nanoparticles were centrifuged at 8000 rpm, washed three times with xylene, dried DMF and absolute ethanol, and then dried in a vacuum oven. The templates were removed from the nanostars, magnetic nanostars, and thiol group-modified nanostars or magnetic nanostars by stirring them three times at 400 rpm in a 10 g L−1 NH4NO3–ethanol solution at 343 K for 3 h; the template-free samples were named NS, M2NS, S-NS and S-M2NS, respectively.
O bonds in 50 mL of N,N-dimethylformamide (DMF) at 323 K with magnetic stirring under an Ar atmosphere for 72 h to form Si–β-CD,24 as shown in Fig. 1(b); the obtained solution was stored at 278 K under dry conditions for further use. 1.45 g of dried nanostars or magnetic nanostars with template (containing 1.0 g of pure SiO2) were added into a reactor with 100 mL xylene and 10 mmol of KH590 (thiol groups). The mixture was refluxed for 24 h at 363 K under a N2 atmosphere, and the powder was collected through a filtration process and washed three times with xylene and ethanol. To achieve the functionalization of the inside pores, the samples were further refluxed in 100 mL xylene with the same volume of DMF solutions containing 0.60 mmol Si–β-CD, 0.45 mmol Si–β-CD, 0.30 mmol Si–β-CD, 0.15 mmol Si–β-CD, 0.10 mmol Si–β-CD and 0.05 mmol Si–β-CD at 363 K for 24 h under a N2 atmosphere, to form different bifunctionalized nanostars, i.e., F0.60NS, F0.45NS, F0.30NS, F0.15NS, F0.10NS and F0.05NS, and magnetic nanostars, i.e., F0.60M2NS, F0.45M2NS, F0.30M2NS, F0.15M2NS, F0.10M2NS, F0.05M2NS, F0.60M1.5NS and F0.60M1NS. These nanoparticles were centrifuged at 8000 rpm, washed three times with xylene, dried DMF and absolute ethanol, and then dried in a vacuum oven. The templates were removed from the nanostars, magnetic nanostars, and thiol group-modified nanostars or magnetic nanostars by stirring them three times at 400 rpm in a 10 g L−1 NH4NO3–ethanol solution at 343 K for 3 h; the template-free samples were named NS, M2NS, S-NS and S-M2NS, respectively.
Fabrication of curcumin loaded bifunctionalized mesoporous silica nanostars and magnetic nanostars
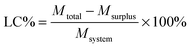
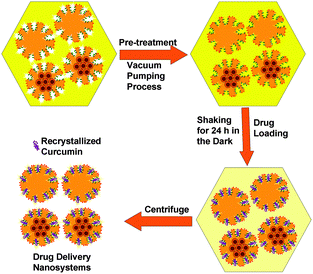
Curcumin loaded bifunctionalized nanostars (Cur@FNS) and (Cur@FMNS) were fabricated through a vacuum pumping assisted recrystallization process, as shown in Fig. 2. Briefly, 100 mg of FNS or FMNS were mixed with 20 mL of a 2.0 × 10−3 M solution of curcumin in ethanol in a reactor after 15 min of ultrasonication, and the suspension was magnetically stirred for 1 h, followed by the dropwise addition of 20 mL of deionized water. The reactor was then vacuum pumped to a vacuum degree of 0.1 MPa for 15 min to remove the air inside the mesoporous channels of FNS or FMNS. The reactor was then shaken for 24 h in the dark in a constant temperature shaker at room temperature. The suspension in the reactor was finally centrifuged, and the upper solution was then diluted with 0.5% sodium lauryl sulfate (SLS) solution and analyzed using UV-vis spectroscopy at the 425 nm wavelength. The quantity of drug remaining could be calculated using a standard curve with a linear range of 0–10 mg L−1. The drug loading capacity (LC%) and entrapment efficiency (EE%) could be calculated using the equations below. The drug delivery nanosystems were collected and dried in a vacuum oven at 303 K overnight.Characterization
The crystal structures of the different samples were determined by XRD (D8 Advance, BRUKER). The FT-IR spectra of the samples were recorded in KBr pellets in the wavenumber range 500–4000 cm−1 using a Fourier transform infrared spectrometer (360, Nicolet Avatar). The surface functionalized molecules were analyzed by XPS (ESCLAB 250, Thermo-VG Scientific). The molecular structures were further studied by solid state 13C and 29Si NMR (400 MHz, AVANCE AV). The thermo-properties of the different samples were tested by TGA and DTA (DTG-60, SHIMADZU) with a maximum temperature of 800 °C in dry air. The mesoporous structures of all samples were confirmed by BET analysis (GEMINI VII 2390, Micromeritics). Microstructures of the different samples were investigated by TEM (G220, Tecnai). Dynamic light scattering (DLS) experiments were performed for the different kinds of drug delivery nanosystems in two kinds of medium, i.e., PBS (pH 7.4, 0.01 M) and simulated cell supernatant (fetal bovine serum (0.05 wt%)–PBS (pH 7.4, 0.01 M)), at constant concentration (6 wt%) in order to investigate the aggregation behavior and stability properties at 25 °C using a zetasizer (Nano-ZS90, Malvern). The drug release behavior of the different samples was studied using a UV-vis spectrophotometer (UV-2550, SHIMADZU).In vitro release properties
In vitro cell testing
Results and discussion
Design of drug delivery nanosystems
Most drug molecules with –OH or –COOH groups, such as curcumin monomers, naturally carry negative charges, from which we realized that endowing the surface with negative groups, such as –SH, could stop the drug from concentrating on the surface of the delivery vehicles. Many drug molecules have benzene ring structures, and we modified the large mesoporous channels with β-CD, which could provide more physical binding sites for the drug molecules, increase drug loading inside the pores and improve the sustained drug release properties. Last but not least, since the curcumin molecular structure is unstable to many chemical or physical conditions, and –NH2 groups might affect the molecular structure of curcumin, we provided specific binding sites for curcumin molecules in order to form stable inclusion complexes. The air inside the mesoporous channels can be removed using the vacuum treatment process, curcumin solution can be driven inside, and the curcumin molecules can be nested in the β-CD molecules. As curcumin shows precipitation properties in water, the water in the curcumin solution could be the driving force for curcumin to precipitate inside the mesopores of the silica during the 24 hour drug loading process. As shown in Fig. S2 (ESI†), the drug delivery properties were improved on increasing the binding sites inside FNS or FMNS. The EE% values were 76.51 ± 6.53% for Cur@F0.60NS and 74.16 ± 6.72% for Cur@F0.60M2NS, compared with 53.82 ± 5.11% for Cur@F0.30NS and 52.96 ± 5.23% for Cur@F0.30M2NS. The control samples were the template-free samples NS and M2NS, with EE% of about 40% and LC% of about 5.5%. As the degree of functionalization with Si–β-CD declined, the drug loading inside FNS and FM2NS depended simply on the recrystallization of the drug, as compared with NS and M2NS. The drug loading process could be divided into two stages: the first stage was the incorporation of soluble curcumin molecules within the β-CD, and the second stage could be the precipitation of curcumin molecules, which “grow” on top of the incorporated curcumin molecules during the overloading process. With more binding sites to combine with the first layer of curcumin, the F0.6M2NS and F0.6NS show better drug loading performance.XRD
XRD patterns for the drug delivery materials are shown in Fig. 3 and Fig. S3(a) (ESI†). As shown in Fig. 3, the inset low-angle XRD plot reveals an almost non-ordered mesoporous structure for NS obtained through the soft-templating process, and after the magnetic core is encapsulated, the non-ordered mesoporous structure is turned into a low-order mesoporous structure, which might be attributed to steric effects between the core structures and the CTA+ micelles during the fabrication process. As shown in Fig. 3, both M2NS and F0.6M2NS show a main peak at 2θ = 35.6°, corresponding to the (311) reflection of the Fe3O4 crystal phase, showing that the magnetic core still remains after the bifunctionalization process. Both F0.6NS and F0.6M2NS did not show obvious peaks corresponding to the β-CD crystal phase, as the Si–β-CD molecules were functionalized on the two kinds of mesoporous silica in the form of mono-molecules. The drug loading properties of the drug delivery nanosystems are shown in Fig. S3(a) (ESI†); through the drug loading process and the driving force of water, curcumin was recrystallized on both F0.6NS and F0.6M2NS, and the prominent peak at 2θ = 17.1°, and the peaks at 2θ = 25.9° and 2θ = 14.4° are still retained in the XRD patterns of Cur@F0.6NS and Cur@F0.6M2NS. Curcumin molecules may be firstly incorporated with Si–β-CD, which may have caused the recrystallized drugs to grow onto the “first layer” of drug molecule complexes during the drug loading process. The complex of curcumin and β-CD molecules would retain the crystalline nature of curcumin.26 However, a lower degree of crystallization was exhibited by Cur@F0.6M2NS compared with Cur@F0.6NS, from which we concluded that the low-ordered mesoporous structure could increase drug dispersion and decrease the amount of the crystalline phase in the nano-formulations.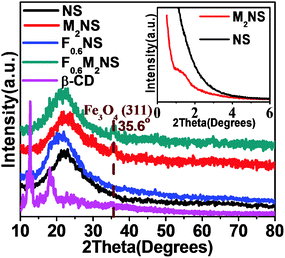 | ||
| Fig. 3 XRD patterns of different samples; the inset image shows low-angle XRD patterns of M2NS and NS. | ||
FTIR analysis
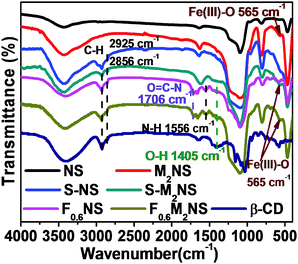
The bifunctionalization process could be confirmed by the change in molecular structure, as shown by the FTIR spectra in Fig. 4. As shown in Fig. 4, all the mesoporous silica samples showed vibrations of Si–O–Si bonds at similar wavenumbers, i.e., 1095 cm−1, 811 cm−1 and 466 cm−1. The template-free S-NS and S-MNS showed vibrations of C–H bonds at 2925 cm−1 and 2856 cm−1 respectively, unlike template-free NS and M2NS, revealing that KH590 had been successfully grafted onto the outer surface of both mesoporous silica samples. The vibrations of N–H bonds at 1556 cm−1 and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N bonds at 1706 cm−1 showed that Si–β-CD had been completely functionalized on both mesoporous silica samples. What's more, the vibration of O–H bonds in β-CD molecules at ∼1400 cm−1 showed that the integrity of the β-CD molecular residues is still maintained during the formation of Si–β-CD molecules and functionalization on mesoporous silica. Both M2NS and F0.6M2NS still contain Fe3O4 cores inside, since the vibration of Fe(III)–O bonds at 565 cm−1 is still present. Drug loading properties and details of the atoms can be observed in Fig. S3(b) (ESI†). The vibration of O
C–N bonds at 1706 cm−1 showed that Si–β-CD had been completely functionalized on both mesoporous silica samples. What's more, the vibration of O–H bonds in β-CD molecules at ∼1400 cm−1 showed that the integrity of the β-CD molecular residues is still maintained during the formation of Si–β-CD molecules and functionalization on mesoporous silica. Both M2NS and F0.6M2NS still contain Fe3O4 cores inside, since the vibration of Fe(III)–O bonds at 565 cm−1 is still present. Drug loading properties and details of the atoms can be observed in Fig. S3(b) (ESI†). The vibration of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N bonds at 1706 cm−1 showed that grafted Si–β-CD is retained on both FMNS and FNS during the drug loading process. The vibrations of the C
C–N bonds at 1706 cm−1 showed that grafted Si–β-CD is retained on both FMNS and FNS during the drug loading process. The vibrations of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H bonds in curcumin molecules at 1627 cm−1, 1510 cm−1 and 1429 cm−1, respectively, showed that the drug had been successfully loaded into F0.6NS and F0.6M2NS, from which we concluded that the curcumin delivery nano-formulations, such as Cur@F0.6NS and Cur@F0.6M2NS, were fabricated successfully.
C–H bonds in curcumin molecules at 1627 cm−1, 1510 cm−1 and 1429 cm−1, respectively, showed that the drug had been successfully loaded into F0.6NS and F0.6M2NS, from which we concluded that the curcumin delivery nano-formulations, such as Cur@F0.6NS and Cur@F0.6M2NS, were fabricated successfully.
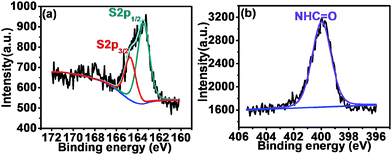
XPS analysis
The surface molecular structures of F0.6NS and F0.6M2NS were confirmed by photoelectron spectroscopy, as shown in Fig. 5. The high resolution S2p spectra exhibited in Fig. 5(a) and Fig. S4(a) (ESI†) revealed the binding energies of the S2p3/2 peak at 164.7 eV and the S2p1/2 peak at 163.5 eV for F0.6NS, and the binding energies of the S2p3/2 peak at 164.5 eV and the S2p1/2 peak at 163.4 eV for F0.6M2NS, which verified the presence of thiol groups and C–S bonds on the surface of both F0.6NS and F0.6M2NS. The high resolution N1s spectra shown in Fig. 5(b) and Fig. S4(b) (ESI†) showed the major amide binding energy of NHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds at 400.0 eV for both F0.6NS and F0.6M2NS, which was attributed to surface functionalized Si–β-CD molecules. According to the results, some of the Si–β-CD molecules were inevitably functionalized onto the surface, due to the open mesoporous structure and the unsaturated –OH sites on the surface. It is notable that no significant signals from the Fe 2p spectrum can be observed in Fig. S4(c) (ESI†) for the surface of F0.6M2NS, and so we believe that Fe3O4 particles were encapsulated inside SiO2 and acted as magnetic cores.
O bonds at 400.0 eV for both F0.6NS and F0.6M2NS, which was attributed to surface functionalized Si–β-CD molecules. According to the results, some of the Si–β-CD molecules were inevitably functionalized onto the surface, due to the open mesoporous structure and the unsaturated –OH sites on the surface. It is notable that no significant signals from the Fe 2p spectrum can be observed in Fig. S4(c) (ESI†) for the surface of F0.6M2NS, and so we believe that Fe3O4 particles were encapsulated inside SiO2 and acted as magnetic cores.
Solid state 13C and 29Si NMR analysis
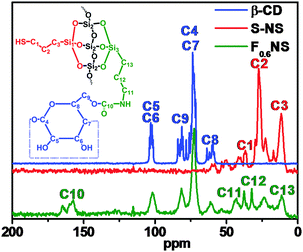
The solid state 13C and 29Si NMR spectra concretely showed the out-inside functionalization process, and F0.6NS was taken as an example. The 13C NMR spectra of β-CD, S-NS and F0.6NS are shown in Fig. 6. In the spectrum of S-NS, the peaks at 12, 27 and 37 ppm were attributed to the C3, C2 and C1 carbon atoms in the surface functionalized HS–(CH2)3– groups, respectively. After further functionalizing S-NS with Si–β-CD, the 13C NMR spectrum of F0.6NS revealed the chemical shifts of the β-CD rings, according to the spectrum of β-CD. Meanwhile, the peaks at 157, 43, 32 and 11 ppm were attributed to the C10, C11, C12 and C13 carbon atoms of the grafted Si–β-CD molecules respectively, which is evidence that β-CD rings were successfully functionalized on the F0.6NS. What's more, the peaks at 37 and 24 ppm were assigned to the C3 and C2 carbon atoms respectively, by which we confirmed the presence of HS–(CH2)3– groups in F0.6NS. In addition, the 29Si NMR spectra of S-NS and F0.6NS revealed the functionalization, as shown in Fig. S5 (ESI†). The peaks observed at −69 ppm for F0.6NS and S-NS were attributed to the presence of T3 structures, corresponding to the modified silane coupling agent. However, the enhanced intensity of the peak at −57 ppm for F0.6NS compared with S-NS was due to the presence of more T2 structures, which reflected the fact that most of the Si–β-CD molecules were functionalized onto S-NS with the breaking of two Si–O–Me bonds, due to the steric hindrance of the β-CD rings. Both F0.6NS and S-NS showed a Q4 NMR shift at −111 ppm, corresponding to Si–(OSi)4. The sharper peak in the spectrum of F0.6NS was associated with increasing amounts of the Q4 structure and decreasing amounts of the Q3 structure of Si(OSi)3(OH) on further functionalization with Si–β-CD molecules, by which we confirmed the success of the out-inside stepwise functionalization.Nitrogen adsorption–desorption isotherms
In order to confirm the alteration of the microstructure during the bifunctionalization and drug loading processes, we choose the M2NS series of samples, the mesoporous structures of which were confirmed by nitrogen adsorption–desorption isotherms. All of the samples were described by type III isotherms for the weak adsorption on the large mesopores, and the hysteresis loops observed at high relative pressures might be attributed to slot–hole stacking by the star shaped mesoporous particles, as shown in Fig. S6 (ESI†). The BET surface areas and aperture parameters shown in Table S1 (ESI†) indicated that outer surface grafted thiol groups hardly influenced the mesoporous structure. When the inner channel was functionalized with Si–β-CD, the BET surface area and the pore volume rapidly decreased, due to the deeper inside modification. After the loading of drugs, the inside mesopores were further filled with drug molecules through the pore bifunctionalization process, the mesoporous structure of the magnetic nanostars still remained, and the drug molecules could be controllably recrystallized and loaded inside the mesopores of the nanocarriers as nano-formulations. What's more, the DTA and TG curves in Fig. S7 (ESI†) and the analysis in the ESI† also confirm the out-inside bifunctionalization process used to fabricate both drug delivery materials, F0.6NS and F0.6M2NS, and to improve the drug loading properties of both drug delivery nanosystems, i.e., Cur@F0.6NS and Cur@F0.6M2NS, as nano-formulations for curcumin.TEM
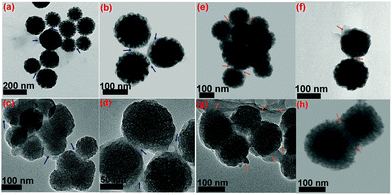
Through the soft-templating conditions, we obtained mono-dispersed star shaped mesoporous silica, as shown in Fig. 7(a) and (b). Through the electrostatic and soft-templating processes, we fabricated nanostar shaped mesoporous silica, as shown in Fig. 7(c), and yolk–shell structures with Fe3O4 cores, as shown in Fig. 7(d) and (e). The Fe3O4 nanoparticles in the water-based ferrofluid had sizes of less than 20 nm, as shown in the inset in Fig. 7(e), and were suitable for encapsulation inside mesoporous silica to form magnetic cores. The particle sizes of NS and M2NS were homogeneously distributed and concentrated in the interval of 90–150 nm. Fig. 8 reveals visually the surface functionalization of NS and M2NS. After the bifunctionalization process, the surface and channels were covered by organic films, and the NS and M2NS particles were wrapped inside shells of organic molecules. These results indicated that NS and M2NS had been successfully functionalized with organic molecules to form F0.6NS and F0.6M2NS. The drug loading results are shown in Fig. 8, from which we concluded that the drugs had recrystallized inside the meso-channels of F0.6NS and F0.6M2NS. However, due to the overloading process, some drug crystals were inevitably grown on the surface, as shown in Fig. 8(e)–(h). The drug delivery nanosystems, i.e., Cur@F0.6NS and Cur@F0.6M2NS, were fabricated as nano-formulations to act as delivery vehicles for curcumin. To further investigate the stability of these drug delivery nanosystems in different physical media, dynamic light scattering (DLS) experiments were carried out for both Cur@F0.6NS and Cur@F0.6M2NS, and the results are shown in Fig. S8 and Table S2 (ESI†), in which the hydrodynamic size was revealed by the Z-average diameter and the stability was revealed by the ζ-potential. In PBS 7.4 medium, the hydrodynamic sizes were 249.09 nm and 299.07 nm for Cur@F0.6NS and Cur@F0.6M2NS respectively, from which we believe that aggregates of 2–3 nanoparticles were most commonly formed in both dispersion systems, according to the particle sizes observed in Fig. 8(e–h). When scattered in cell supernatant, with electrostatic forces due to the protein molecules, the hydrodynamic sizes of both drug nanocarriers increased while the polydispersity index (PDI) values decreased, as a result of the enhanced scattering properties of the protein molecules, which might increase the bioavailability under physical conditions. The absolute ζ-potential values decreased in cell supernatant compared with pure PBS 7.4 medium, due to the increased degree of hydration and increased hydrodynamic size caused by the protein molecules present under the simulated physical conditions.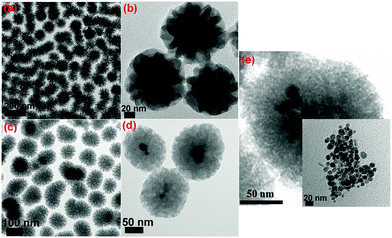 | ||
| Fig. 7 TEM images of (a) and (b) NS; (c), (d) and (e) M2NS; the inset image in (e) shows the Fe3O4 nanoparticles in the water-based ferrofluid. | ||
In vitro cell testing
The drug delivery materials, such as NS, M2NS, F0.6NS and F0.6M2NS, were tested for cell apoptosis with the Hoechst 33342 staining experiment, and hemolysis to evaluate the cytotoxicity and biocompatibility before and after bifunctionalization. From the hemolysis rates shown in Fig. 9, we can see that the NS and M2NS exhibit obvious hemolysis properties at high concentrations of 1000–2000 μg mL−1; the hemolysis rates were measured as over 10%. The hemolysis rate was lowered through the bifunctionalization process, since the hemolysis rates of both F0.6NS and F0.6M2NS were less than 10% when the concentrations were 1000–2000 μg mL−1, from which we confirmed that the bifunctionalization could increase the biocompatibility of the drug nanocarriers with blood cells. Some of the Si–β-CD molecules were inevitably functionalized on the silanol surface, reducing and modifying the silanol groups with β-CD rings and saccharide hydroxyl groups, which could reduce the toxicity to the blood cells.27 The cell apoptosis results are shown in Fig. S9 and S10 (ESI†), and there was no obvious cytotoxicity of the drug delivery materials towards normal cells or cancer cells.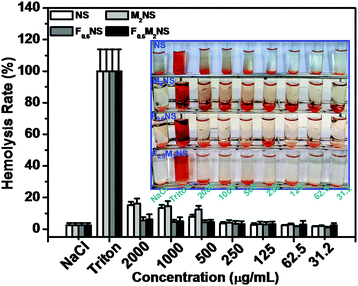 | ||
| Fig. 9 Hemolysis assays for NS, M2NS, F0.6NS and F0.6M2NS at concentrations from 31.2 μg mL−1 to 2000 μg mL−1. | ||
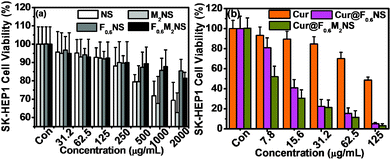
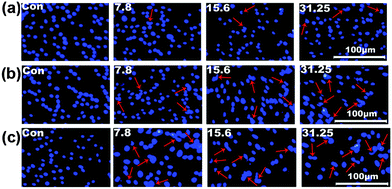
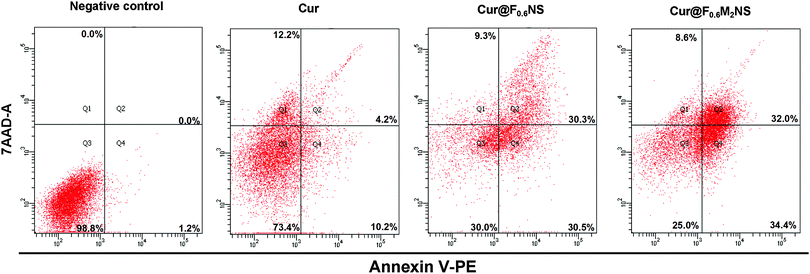
The cell viability testing results for 293T cells are shown in Fig. S11 (ESI†), and those for SK-HEP1 cells are shown in Fig. 10(a), in order to evaluate the cytotoxicity of the drug delivery nanocarriers. The cytotoxicity of both the NS and M2NS samples increased when the concentrations increased to above 500 μg mL−1, which might be attributed to their star-shaped structures and cluster surfaces. However, after the bifunctionalization process, both the F0.6NS and F0.6M2NS samples retained high cell viability for both 293T cells and SK-HEP1 cells, and even at high concentrations of 2000 μg mL−1, the cell viability was measured as over 80%, revealing improved biocompatibility and lower toxicity to cells. Additionally, the SK-HEP1 cell viability of F0.6NS and F0.6M2NS was measured as over 95% through flow cytometry at concentrations of 125 and 500 μg mL−1, as shown in Fig. S12 (ESI†). Meanwhile, the cytotoxicities of different drug nano-formulations of curcumin were evaluated using cancer cells, such as SK-HEP1 cells as shown in Fig. 10(b), and HepG2 cells as shown in Fig. S13 (ESI†). However, pure curcumin did not show any significant toxicity to HepG2 cells; the cell viability was measured as 100% at the given concentrations, and the IC50 value was 121.3 μg mL−1 for SK-HEP1 cells, which reveals the poor bioavailability and low uptake properties of free curcumin. The IC50 values of Cur@F0.6NS and Cur@F0.6M2NS were 13.8 μg mL−1 and 8.57 μg mL−1 respectively for SK-HEP1 cells, and 10.9 μg mL−1 and 7.21 μg mL−1 respectively for HepG2 cells. The toxicity was increased 9-fold for Cur@F0.6NS nano-formulations, and 14-fold for Cur@F0.6M2NS nano-formulations, verifying the improved bioavailability of the nano-formulations, due to the novel bifunctionalized magnetic star shaped mesoporous silica. The cell apoptosis results in Fig. 11 reveal the cell apoptosis during incubation with different curcumin nano-formulations, such as free curcumin, Cur@F0.6NS and Cur@F0.6M2NS; the cell apoptosis responses to Cur@F0.6NS and Cur@F0.6M2NS were obviously increased compared with free curcumin, even at low concentrations of 7.8 μg mL−1, showing visually the increased toxicity of the curcumin nano-formulations to SK-HEP1 cells. Last but not least, the apoptosis rates were significantly increased for both designed nanocarriers compared with free curcumin, as shown in Fig. 12. The early apoptosis rates were 10.2%, 30.5% and 34.4% for free curcumin, Cur@F0.6NS and Cur@F0.6M2NS respectively, and the late apoptosis rates were 4.2%, 30.3% and 32.0% respectively, showing the enhanced ability of the designed nanocarriers to kill SK-HEP1 cells.
 | ||
| Fig. 12 Apoptosis induction in the SK-HEP1 cell line for 48 h by different drug nano-formulations, namely curcumin, Cur@F0.6NS and Cur@F0.6M2NS, with curcumin concentration of 31.25 μg mL−1. | ||
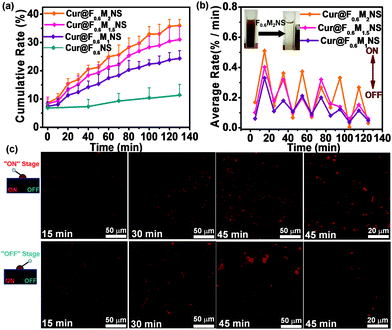
The cellular uptake was characterized by confocal fluorescence microscopy, as shown in Fig. S16 (ESI†). However, the free curcumin did not show any cellular uptake effect after 1 h and 4 h, revealing the low bioavailability and uptake properties of free curcumin. The nano-sized curcumin formulations, Cur@F0.6NS and Cur@F0.6M2NS showed increased cellular uptake of curcumin, from which we concluded that the novel delivery nanosystems could increase the bioavailability and the toxicity of the drug towards cancer cells. When we fabricated bifunctionalized nanostars with Fe3O4 magnetic cores, the release of curcumin in 0.5% SLS could be adjusted by an AMF, and the payloads of Fe3O4 nanoparticles inside the bifunctionalized nanostars are shown in Fig. 13(a) and (b). As we increased the payload of Fe3O4 nanoparticles, the drug carriers became increasingly responsive to the “on–off” AMF, as the average release rate during the “on” stage increased from Cur@F0.6M1NS to Cur@F0.6M2NS. The inset in Fig. 13(b) also reveals the rapid magnetic collection of F0.6M2NS in water. It is worth noting that these samples retained their sensitivity to the AMF, even after 100 min, the 10th “on–off” alternation cycle. The ability to control these drug delivery nanosystems with an AMF could increase the suspension and release of curcumin in the 0.5% SLS solution, and the control of the release by the alternating magnetic field was attributed to intense local heat due to magnetic–thermal conversion,28 by which the bioavailability and suspension of curcumin might be improved under magnetically controlled conditions. Furthermore, for the “on” stage of the alternating magnetic field, the fluorescence signal was obviously increased compared with the “off” stage during incubation with SK-HEP1 cells, as shown in Fig. 13(c). Significant cellular uptake properties were observed during the “on” stage after 45 min. As an effect of alternating magnetic fields, the signal increase depended on the stimulation time. After 45 min, the intercellular release was significantly different compared with incubation for 30 and 15 min, showing that the cellular uptake and bioavailability had been improved. These results indicate that the magnetism responsive nanocarriers exhibited a smart and sensitive response to alternating magnetic fields, not only in the in vitro release medium, but also between the cells. With magnetic cores, which are heat mediators suitable for localized or targeted hyperthermia therapy,29 the bifunctionalized nanostars could be applied in MRI and diagnosis as well.
Conclusions
In this work, we have explored the fabrication of two kinds of bifunctionalized nanostars for the delivery of nano-formulations of the curcumin drug, in which the drug delivery nanocarriers could effectively increase the biocompatibility and decrease the cytotoxicity to cells. Meanwhile, these drug nano-formulations could significantly increase the cytotoxicity to cancer cells, such as SK-HEP1 cells and HepG2 cells, compared with free curcumin, in order to kill them more effectively. The cellular uptake properties and bioavailability of these curcumin nano-formulations were increased in SK-HEP1 cells. With the use of magnetic cores, the drug delivery nanostars showed a sensitive response to magnetic fields, and exhibited excellent controllability of drug release both in vitro and intercellularly. Bifunctionalization combined with magnetic fabrication has immense potential in the drug delivery field and in biomedicine.Acknowledgements
The work was supported by the National Natural Science Foundation (P. R. China, No. 31101854, 61427807 and 21207041), and Fujian Province Introduction of Major Research and Development Institution Funding Project (2012I2014).Notes and references
- K. Zhang, L. L. Xu, J. G. Jiang, N. Calin, K. F. Lam, S. J. Zhang, H. H. Wu, G. D. Wu, B. Albela, L. Bonneviot and P. Wu, J. Am. Chem. Soc., 2013, 135, 2427–2430 CrossRef CAS PubMed.
- (a) M. Wu, L. Y. Kong, K. W. Wang, R. H. Jin, T. Y. Cheng and G. H. Liu, Catal. Sci. Technol., 2015, 5, 1750–1757 RSC; (b) X. Du and S. Z. Qiao, Small, 2015, 11, 392–413 CrossRef CAS PubMed.
- (a) Y. F. Zhu and C. L. Tao, RSC Adv., 2015, 5, 22365–22372 RSC; (b) L. Xiong, X. Du, B. Y. Shi, J. X. Bi, F. Kleitz and S. Z. Qiao, J. Mater. Chem. B, 2015, 3, 1712–1721 RSC.
- J. P. Yang, W. Y. Chen, D. K. Shen, Y. We, X. Q. Ran, W. Teng, J. W. Fan, W. X. Zhang and D. Y. Zhao, J. Mater. Chem. A, 2014, 2, 11045–11048 CAS.
- C. L. Tao, Y. F. Zhu, Y. Xu, M. Zhu, H. Morita and N. Hanagata, Dalton Trans., 2014, 43, 5142–5150 RSC.
- F. Liu, J. N. Wang, P. L. Huang, Q. Zhang, J. T. Deng, Q. Y. Cao, J. T. Jia, J. H. Cheng, Y. P. Fang, D. Y. B. Deng and W. Y. Zhou, J. Mater. Chem. B, 2015, 3, 2206–2214 RSC.
- P. L. Huang, J. N. Wang, S. T. Lai, F. Liu, N. Ni, Q. Y. Cao, W. Liu, D. Y. B. Deng and W. Y. Zhou, J. Mater. Chem. B, 2014, 2, 8616–8625 RSC.
- G. Astray, C. Gonzalez-Barreiro, J. C. Mejuto, R. Rial-Otero and J. Simal-Gándara, Food Hydrocolloids, 2009, 23, 1631–1640 CrossRef CAS.
- (a) K. J. Waleczek, H. M. Marques, B. Hempe and P. C. Schmidt, Eur. J. Pharm. Biopharm., 2003, 55, 247–251 CrossRef CAS PubMed; (b) V. A. Marcolino, G. M. Zanin, L. R. Durrant, M. D. Benassi and G. Matioli, J. Agric. Food Chem., 2011, 59, 3348–3357 CrossRef CAS PubMed; (c) C. Jantarat, P. Sirathanarun, S. Ratanapongsai, P. Watcharakan, S. Sunyapong and A. Wadu, Trop. J. Pharm. Res., 2014, 13, 1215–1223 CrossRef CAS.
- (a) N. M. Khashab, A. Trabolsi, Y. A. Lau, M. W. Ambrogio, D. C. Friedman, H. A. Khatib, J. I. Zink and J. F. Stoddart, Eur. J. Org. Chem., 2009, 1669–1673 CrossRef CAS; (b) D. P. Ferris, Y. L. Zhao, N. M. Khashab, H. A. Khatib, J. F. Stoddart and J. I. Zink, J. Am. Chem. Soc., 2009, 131, 1686–1688 CrossRef CAS PubMed; (c) S. Angelos, Y. W. Yang, N. M. Khashab, J. F. Stoddart and J. I. Zink, J. Am. Chem. Soc., 2009, 131, 11344–11346 CrossRef CAS PubMed; (d) J. Croissant, A. Chaix, O. Mongin, M. Wang, S. Clément, L. Raehm, J. O. Durand, V. Hugues, M. Blanchard-Desce, M. Maynadier, A. Gallud, M. Gary-Bobo, M. Garcia, J. Lu, F. Tamanoi, D. P. Ferris, D. Tarn and J. I. Zink, Small, 2014, 10, 1752–1755 CrossRef CAS PubMed.
- (a) M. Shoji, K. Nakagawa, A. Watanabe, T. Tsuduki, T. Yamada, S. Kuwahara, F. Kimura and T. Miyazawa, Food Chem., 2014, 151, 126–132 CrossRef CAS PubMed; (b) C. S. Mangolim, C. Moriwaki, A. C. Nogueira, F. Sato, M. L. Baesso, A. M. Neto and G. Matioli, Food Chem., 2014, 153, 361–370 CrossRef CAS PubMed.
- (a) M. Salem, S. Rohani and E. R. Gillies, RSC Adv., 2014, 4, 10815–10829 RSC; (b) H. Yan, C. Teh, S. Sreejith, L. L. Zhu, A. Kwok, W. Fang, X. Ma, K. T. Nguyen, V. Korzh and Y. L. Zhao, Angew. Chem., Int. Ed., 2012, 51, 8373–8377 CrossRef CAS PubMed; (c) H. Hatchera, R. Planalpb, J. Chob, F. M. Tortia and S. V. Tortic, Cell. Mol. Life Sci., 2008, 65, 1631–1652 CrossRef PubMed.
- Y. Hao, Y. J. Pan, N. Nitin and R. V. Tikekar, LWT–Food Sci. Technol., 2014, 58, 667–671 CrossRef.
- S. P. Singh, M. Sharma and P. K. Gupta, Int. J. Biol. Macromol., 2015, 74, 162–170 CrossRef CAS PubMed.
- C. L. Tong, U. H. Stroeher, M. H. Brownb and C. L. Raston, RSC Adv., 2015, 5, 7953–7958 RSC.
- A. Saithongdee, N. Praphairaksit and A. Imyim, Sens. Actuators, B, 2014, 202, 935–940 CrossRef CAS.
- (a) J. R. Lou, X. X. Zhang, J. Zheng and W. Q. Ding, Anticancer Res., 2010, 30, 3249–3255 CAS; (b) L. Baum and A. Ng, J. Alzheimer's Dis., 2004, 6, 367–377 CAS.
- M. Massaro, S. Riela, P. L. Meo, R. Noto, G. Cavallaro, S. Miliotob and G. Lazzara, J. Mater. Chem. B, 2014, 2, 7732–7738 RSC.
- F. Liu, H. Zhang, Q. Y. Cao, X. H. Xiang, L. Q. Wang, T. He, W. Liu, Y. P. Fang, D. Y. B. Deng and W. Y. Zhou, RSC Adv., 2014, 4, 8918–8921 RSC.
- H. H. Tønnesen and J. Karlsen, Z. Lebensm.-Unters. Forsch., 1985, 180, 402–404 CrossRef.
- A. F. Wang, F. Muhammad, W. X. Qi, N. Wang, L. Chen and G. S. Zhu, ACS Appl. Mater. Interfaces, 2014, 6, 14377–14383 CAS.
- (a) D. Jin, K. W. Park, J. H. Lee, K. Song, J. G. Kim, M. L. Seo and J. H. Jung, J. Mater. Chem., 2011, 21, 3641–3645 RSC; (b) R. K. Gangwar, G. B. Tomar, V. A. Dhumale, S. Zinjarde, R. B. Sharma and S. Datar, J. Agric. Food Chem., 2013, 61, 9632–9637 CAS.
- F. Liu, J. N. Wang, Q. Y. Cao, H. D. Deng, G. Shao, D. YB. Deng and W. Y. Zhou, Chem. Commun., 2015, 51, 2357–2360 RSC.
- C. Abbehausen, A. L. B. Formiga, E. Sabadini and I. V. P. Yoshida, J. Braz. Chem. Soc., 2010, 21, 1867–1876 CrossRef CAS.
- (a) S. Jambhrunkar, S. Karmakar, A. Popat, M. H. Yu and C. Z. Yu, RSC Adv., 2014, 4, 709–712 RSC; (b) A. Popat, S. Karmakar, S. Jambhrunkar, C. Xu and C. Z. Yu, Colloids Surf., B, 2014, 117, 520–527 CrossRef CAS PubMed.
- H. K. Syed and K. K. Peh, Lat. Am. J. Pharm., 2013, 32, 52–59 CAS.
- (a) Y. S. Lin and C. L. Haynes, J. Am. Chem. Soc., 2010, 132, 4834–4842 CrossRef CAS PubMed; (b) I. I. Slowing, C. W. Wu, J. L. Vivero -Escoto and V. S. Y. Lin, Small, 2009, 5, 57–62 CrossRef CAS PubMed.
- H. Wang, J. H. Yi, S. Mukherjee, P. Banerjee and S. Q. Zhou, Nanoscale, 2014, 6, 13001–13011 RSC.
- A. Hervault and N. T. K. Thanh, Nanoscale, 2014, 6, 11553–11573 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5tb02184g |
| ‡ Both authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |