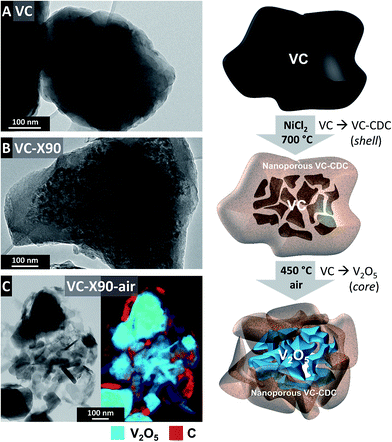
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Vanadium pentoxide/carbide-derived carbon core–shell hybrid particles for high performance electrochemical energy storage†
Marco
Zeiger
ab,
Teguh
Ariyanto
c,
Benjamin
Krüner
ab,
Nicolas J.
Peter
d,
Simon
Fleischmann
b,
Bastian J. M.
Etzold
*ce and
Volker
Presser
*ab
aINM – Leibniz Institute for New Materials, 66123 Saarbrücken, Germany. E-mail: volker.presser@leibniz-inm.de
bDepartment of Materials Science and Engineering, Saarland University, 66123 Saarbrücken, Germany
cFriedrich-Alexander Universität Erlangen-Nürnberg, Lehrstuhl für Chemische Reaktionstechnik, 91058 Erlangen, Germany
dMax-Planck Institut für Eisenforschung GmbH, 40237 Düsseldorf, Germany
eTechnische Universität Darmstadt, Ernst-Berl-Institut für Technische und Makromolekulare Chemie, 64287 Darmstadt, Germany. E-mail: etzold@tc1.tu-darmstadt.de
First published on 31st October 2016
Abstract
A novel, two step synthesis is presented combining the formation of carbide-derived carbon (CDC) and redox-active vanadium pentoxide (V2O5) in a core–shell manner using solely vanadium carbide (VC) as the precursor. In a first step, the outer part of VC particles is transformed to nanoporous CDC owing to the in situ formation of chlorine gas from NiCl2 at 700 °C. In a second step, the remaining VC core is calcined in synthetic air to obtain V2O5/CDC core–shell particles. Materials characterization by means of electron microscopy, Raman spectroscopy, and X-ray diffraction clearly demonstrates the partial transformation from VC to CDC, as well as the successive oxidation to V2O5/CDC core–shell particles. Electrochemical performance was tested in organic 1 M LiClO4 in acetonitrile using half- and asymmetric full-cell configuration. High specific capacities of 420 mA h g−1 (normalized to V2O5) and 310 mA h g−1 (normalized to V2O5/CDC) were achieved. The unique nanotextured core–shell architecture enables high power retention with ultrafast charging and discharging, achieving more than 100 mA h g−1 at 5 A g−1 (rate of 12C). Asymmetric cell design with CDC on the positive polarization side leads to a high specific energy of up to 80 W h kg−1 with a superior retention of more than 80% over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles and an overall energy efficiency of up to 80% at low rates.
000 cycles and an overall energy efficiency of up to 80% at low rates.
1. Introduction
Supercapacitors are electrochemical energy storage devices, which present ultrafast charge–discharge rates leading to higher power compared to conventional batteries, but suffer from relatively low specific energies.1–4 The high specific power of ∼10 kW kg−1 benefits applications with charge- and discharge rates in the order of seconds and minutes, such as flashlights, high power machinery, or emergency doors in airplanes, to name just a few.2,5 Depending on the energy storage mechanism, we can differentiate between two types of supercapacitors, namely electrical double-layer capacitors (EDLCs) that store energy by ion electrosorption, and pseudocapacitors that accomplish a capacitor-like charge-voltage behavior by fast redox reactions.6 Today, most EDLCs employ activated carbon (AC) electrodes with surface areas between 1500 and 2000 m2 g−1, leading to typical capacitance values of 100–200 F g−1 and specific energies of up to ca. 20 W h kg−1, depending on the electrolyte and the cell configuration.6 By virtue of these values, the energy storage capacity of supercapacitors is significantly smaller than conventional batteries (e.g., lithium ion batteries: >100 W h kg−1).6 A facile way to enhance the electrochemical performance is to increase the specific surface area of the electrode material and to optimize the pore size distribution.7 This is particularly effective for a material with very high pore tunability, as presented in the case of carbide-derived carbons (CDCs).8 However, this strategy is limited by the minimum pore size required for ion accessibility and the charge screening ability of carbon.7,9,10 Further enhancements of the energy storage capacity can be accomplished by employing redox-active materials for faradaic charge transfer.11,12 The latter can be either in the form of fast redox processes of surface groups, ion intercalation in the electrode material, or redox reactions of the electrolyte.13,14 Depending on the charge-voltage profile of the involved faradaic reaction(s), one differentiates between capacitor-like (i.e., pseudocapacitive) or battery-like behavior.15 Pseudocapacitive materials, exhibiting a constant ratio of charge and voltage (i.e., capacitance, unit: farad), are, for example, ruthenium oxide,16 manganese oxide,17 or MXene.18 Battery-like systems, exhibiting distinct redox peaks in the cyclic voltammogram, are, for example, quinone surface groups19,20 or many metal oxides working as cathodic lithium-intercalation hosts (such as LiFePO4, LiCoO2, LiMn2O4, and LiFeSO4F; ref. 21).A very promising electrochemical system employs the hybridization of carbon materials with vanadium pentoxide (V2O5).22–26 By this way, the beneficial electrical conductivity and nanotextured surface of carbon is complemented by the high, battery-like charge storage capacity of vanadia with a theoretical capacity of 147 mA h g−1 for intercalation of 1 M of Li in the V2O5 structure.27 Several studies have investigated vanadium pentoxide as a cathode material in lithium ion batteries, exhibiting high capacities between 350 and 750 mA h g−1.28–30 Furthermore, other types of vanadia in different oxidation states can be used in batteries, such as VO2,31 and also dissolved as an electrolyte in redox-flow batteries.32 In a previous study, we used atomic layer deposition (ALD) for highly controllable decoration of carbon onions with V2O5, achieving defined thickness, morphology, and crystallinity of the metal oxide phase.33 A high specific energy (38 W h kg−1) was achieved for a full-cell using V2O5/carbon onion vs. activated carbon (asymmetric cell), which is ca. 2-times higher than that of standard activated carbon in organic electrolytes.33 Even higher values for the specific energy were found for interpenetrating networks of CNTs and V2O5 nanowires (40 W h kg−1)22 and vanadia-coated reduced graphene oxide sheets (76 W h kg−1).34 While promising, the practical adaptation of microfabrication and ALD remains limited and more facile synthesis routes for V2O5/carbon hybrid materials remain in high demand.
The combination of redox-active metal oxides with conductive and porous carbons in a hybrid device can be achieved by mechanical mixing (thus exhibiting strongly reduced conductivity), or the decoration of the carbon with metal oxides. For the latter, especially when using highly porous carbons, issues of pore clogging may arise, leading to a strong decrease in capacitance and specific energy. These complications are avoided by not coating the carbon with the metal oxide, but rather fabricating a metal oxide core with a porous carbon shell. Inspired by carbon only core–shell architectures, which can be obtained for CDCs,35,36 we present a novel two-step synthesis for V2O5/CDC composites in a core–shell arrangement. Our approach requires only one precursor (vanadium carbide), which is first partially transformed to nanoporous CDC by chlorine treatment of carbide, and then partially calcined, yielding core–shell V2O5/CDC particles. The chlorination step was needed as it is an effective way to transform a metal carbide to carbon. The nanoporous carbon shell makes the vanadium pentoxide core accessible for ion intercalation and works as a conductive additive. This was accomplished by a high level of control over the synthesis steps, which prevented a completion of both the chlorination and the oxidation processes. Rigorous structural and electrochemical characterization was carried out to establish the high energy storage capacity of the material in organic electrolytes. The combination of carbon and vanadia is of mutual benefit: carbon contributes electrical conductivity (and, to a lesser extent, double-layer capacitance) and vanadia provides a large energy storage capacity. However, the design of the two phases and their nanoscopic arrangement are of high importance for the resulting electrochemical performance. As our data will show, the firm of transformed VC-derived V2O5 and VC-CDC enables attractive electrochemical performances.
2. Materials and methods
V2O5/porous carbon core–shell particles were synthesized using a two-step approach: (1) VC/CDC core–shell particles are produced by etching vanadium from the outside to the inside of VC particles with a remaining VC core. (2) The residual VC core inside the CDC matrix is calcined by oxidation in synthetic air, resulting in V2O5/CDC core–shell particles. These two steps are explained in detail in the next sections.2.1 Synthesis of vanadium carbide/carbide-derived carbon core–shell particles
Vanadium carbide (VC)/CDC core–shell particles were synthesized by reactive extraction of vanadium using in situ generation of chlorine. 1 g VC powder (purity 99.9%, <2 µm, Sigma Aldrich) was thoroughly mixed with NiCl2·6H2O (purity 99.95%, Alfa Aesar) and filled into a graphite crucible placed in an isothermal zone of the reactor (Ø = 32 cm, length = 1 m). The reactor was evacuated to 0.01 mbar and then heated to 700 °C at a heating rate of 2.5 °C min−1. After a holding period of 3 h at this temperature, the reactor was cooled to room temperature under a helium purge. The resulting product was then poured into 500 mL of 1 M HCl and the solution was vigorously stirred using a magnetic stir bar. After filtration, the acid washing was repeated with similar procedures to ensure the removal of nickel species. The product was finally washed using distilled water until neutral pH and then dried in an oven (60 °C) overnight.To achieve incomplete carbide-to-CDC conversion, we used molar ratios of NiCl2·6H2O-to-VC of 1.4, 1.8, and 2.7. It is assumed that chlorine (Cl2) is released during vacuum decomposition of NiCl2 and reacts with VC in a stoichiometric reaction to produce VCl4viaeqn (1) and (2).
| [NiCl2·6H2O]solid → [Ni]solid + [Cl2]gas + [6H2O]gas | (1) |
| [VC]solid + [2Cl2]gas → [C]solid + [VCl4]gas | (2) |
Based on these reactions and assuming that all chlorine reacts with carbide the theoretical conversion (Xtheo.) can be derived from eqn (3) assuming full reaction of VC with Cl2 release.
 | (3) |
To produce a reference material through the “classical” and not in situ chlorine generation route, VC was exposed to chlorine gas diluted in helium at 700 °C for 5 h, as described elsewhere.63 The resulting material is labelled VC-CDC.
2.2 Synthesis of V2O5/carbide-derived carbon core–shell particles
Calcination of the VC/CDC particles was performed at 450 °C for 30 min in a VG Scienta furnace using synthetic air. Additionally, VC particles without prior chlorine gas treatment were oxidized at different temperatures (450, 500, and 600 °C) for 4 h to produce V2O5 without an additional carbon shell.2.3 Chemical and structural characterization
The chemical composition of all samples was measured by energy-dispersive X-ray analysis (EDX) using a X-Max-150 detector from Oxford Instruments in a JSM-7500F (JEOL) scanning electron microscope. Spectra were taken at ten different positions with an acceleration voltage of 10 kV.Transmission electron microscopy (TEM) was carried out with a JEOL 2100F microscope using 200 kV acceleration voltage. Powders were dispersed in isopropanol, tip sonicated for 10 s and dropcast on a copper grid with a lacey carbon film (Gatan) support.
Chemical composition maps were acquired by energy filtered transmission electron microscopy (EF-TEM) using a FEI Titan Themis 60-300 at an acceleration voltage of 300 kV. Electron energy loss spectra (EELS) were recorded using a Gatan GIF Quantum ERS energy filter at an energy resolution of ∼1.1 eV. From the acquired EELS the edge onsets of the carbon K-edge, the vanadium L-edge and the oxygen K-edge were determined and the three-window method applied to acquire the respective elemental maps. A slit width of 10 eV was utilized for the pre- and post-edge images. Since the vanadium and oxygen edges overlap, the exact same pre-edge windows were taken for both images. More information can be found in the ESI.†
Thermogravimetric analysis (TGA) was carried out with a TG 209 F1 Libra system (Netzsch). Samples were heated up to 900 °C in synthetic air with a rate of 20 °C min−1 as well as isothermally at 450 °C for 30 min. The sample mass ranged between 10 mg and 20 mg.
The nitrogen gas sorption analysis at −196 °C was carried out with a Quantachrome Autosorb iQ system and the calculations were performed with ASiQwin-software 3.0. The samples were degassed at 100 °C for 1 h and subsequently heated to 150 °C and kept at this temperature for up to 20 h at a relative pressure of 0.1 Pa to remove volatile molecules from the surface. The relative pressure with nitrogen was 5 × 10−7 to 1.0 in 76 steps. The specific surface area (SSA) was calculated using the Brunauer–Emmett–Teller equation (BET) in the linear regime of the measured isotherms, typically 3 × 10−2 to 2 × 10−1 (relative pressure), with a Quantachrome Autosorb 6B.64
Raman spectra were recorded with a Renishaw inVia Raman Microscope equipped with a Nd–YAG laser (532 nm). A 50× objective was used with a power of 0.02 mW at the surface of the sample. The spectra of CDC samples were recorded with 10 accumulations and 20 s acquisition time. Vanadium pentoxide and vanadium carbide spectra were recorded with 1 accumulation and 10 s acquisition as well as in two separate measurements for the full wavenumber range (0–4000 cm−1). All spectra were normalized and background corrected by subtracting a linear baseline.
X-ray diffractograms were collected with a Bruker D8 Discover diffractometer using Cu-Kα radiation (0.154 nm) with a step size of 0.02° and a measurement time of 1 s per step. The system was calibrated with a corundum standard. The free-standing PTFE-bound electrodes were placed on a sapphire single crystal for the measurement.
2.4 Electrochemical characterization
For electrochemical characterization, free-standing, polymer-bound electrodes were used. The powder materials were dispersed in isopropanol and stirred in a mortar until most of the isopropanol was evaporated and a carbon slurry was obtained. For all samples, 10 mass% PTFE (60 mass% aqueous solution, Sigma Aldrich) was added and mixed in a mortar until a dough-like paste was formed. For the electrodes with pure V2O5 (VC-500) an additional conductive additive (see also the ESI, Table S1†), in this case 25 mass% VC-CDC was added to achieve the same amount of carbon as in the core–shell electrodes. Using a rolling machine (MTI HR01, MTI Corp.) electrodes with a thickness of 60 µm (±10 µm) were fabricated and dried overnight at 90 °C and 20 mbar.Electrochemical characterization was carried out in 1 M LiClO4 in acetonitrile (ACN) from BASF. For all measurements, a two- and three-electrode setup was used, corresponding to full- and half-cell configuration, respectively. The electrodes were punched out to obtain discs with a diameter of 6 mm (1–3 mg) and were separated by a glass-fiber disc with a diameter of 13 mm (type GF/A, GE Healthcare). The electrode/separator/electrode arrangement was compressed between two carbon-coated aluminum discs (diameter 12 mm, type Zflo 2653, Coveris Advanced Coatings) using spring-loaded titanium pistons, sealed by a polyether ether ketone (PEEK) body. The cells were dried at 90 °C and 20 mbar before they were put in an argon-filled glove box (MBraun Labmaster 130; O2, H2O <1 ppm). The cells were vacuum back-filled with a syringe containing the electrolyte. Full-cell measurements were carried out in an asymmetric configuration with a charge-balanced counter VC-CDC electrode by measuring the discharge capacity at 0.05 A g−1 in a half-cell setup for both, working and counter electrodes, and adjusting the mass of the VC-CDC electrode. In the half-cell setup, a ca. five times oversized AC counter electrode was used together with a reference electrode; the latter was PTFE-bound activated carbon YP-50F from Kuraray.
Electrochemical characterization was performed using a VSP300 and VMP300 potentiostat/galvanostat from Bio-Logic in cyclic voltammetry (CV) and galvanostatic mode with potential limitation (GCPL). In the half-cell configuration a potential window from −1.2 V to +1.2 V vs. carbon was used. For data obtained with cyclic voltammetry, a scan rate of 1 mV s−1 was chosen to clearly identify the redox peaks. In galvanostatic mode with potential limitation (GCPL), specific currents up to 20 A g−1 were applied. All specific current values were normalized to the active mass of the working electrode (i.e., without the binder). Every cycle was repeated 2 times, followed by a 10 s resting time. The specific capacity of the working electrode in the half-cell configuration was calculated according to eqn (4), using the data from GCPL.
 | (4) |
The specific energy (Especific) and specific power (Pspecific) of the asymmetric full-cells (two electrodes) were calculated using eqn (5) and (6):
 | (5) |
| Pspecific = Especific/(t2 − t1) | (6) |
Long-time stability was measured by galvanostatic cycling at 1 A g−1 from 0 V to 2.5 V cell voltage for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times. The energy efficiencies, as well as the energy retention, were calculated dividing the respective value from discharging by the value from the charging part of the curve and are presented in percent.
000 times. The energy efficiencies, as well as the energy retention, were calculated dividing the respective value from discharging by the value from the charging part of the curve and are presented in percent.
3. Results and discussion
3.1 Structure, chemical composition, and porosity
In this study, a novel two-step process is presented, which can be used for the synthesis of vanadium oxide combined with porous carbide-derived carbon hybrid structures in a core–shell arrangement: (1) VC/CDC core–shell particles are produced by etching vanadium from the outside to the inside of VC particles with a remaining VC core. (2) The residual VC core inside the CDC matrix is calcined by oxidation in synthetic air, resulting in V2O5/CDC core–shell particles. The resulting samples are labelled VC-X70, VC-X90, and VC-X100 for a theoretical conversion of 70%, 90%, and 100%. For additional calcination the samples were labelled VC-X70-air, VC-X90-air, and VC-X100-air. Complete transformation from VC to CDC (i.e., VC-CDC) was achieved by conventional chlorine gas treatment at 700 °C, resulting in highly disordered carbon (Fig. 1A). Minor amounts of VC are still visible in VC-CDC (Fig. 1A) but not quantitatively significant according to the chemical analysis (Table 1). For the synthesis of VC/CDC core–shell particles with different ratios of VC to CDC, the amount of NiCl2 was varied for in situ chlorine formation during thermal treatment. As expected from the stoichiometric calculation presented in eqn (3), VC-X70 had the largest amount of VC in the composite with 40.2 ± 5.3 mass%. Larger amounts of NiCl2 led to lower VC contents of 29.0 ± 7.5 mass% and 6.9 ± 2.4 mass% for VC-X90 and VC-X100, respectively (Table 1). Although a stoichiometric surplus of chlorine in VC-X100 should result in complete transformation to CDC, TEM analysis still shows a small core region of residual VC. We explain this by a reduced vanadium etching rate because of the graphitic carbon layers wrapped around the carbide domains.37| C (mass%) | V (mass%) | O (mass%) | Cl (mass%) | Ni (mass%) | VC (mass%) | V2O5 (mass%) | |
|---|---|---|---|---|---|---|---|
| VC | 22.0 ± 0.5 | 77.6 ± 0.7 | n.d. | n.d. | n.d. | 95.9 ± 1.1 | |
| VC-X70 | 60.6 ± 4.1 | 32.5 ± 4.7 | 6.3 ± 1.1 | 0.6 ± 0.1 | n.d. | 40.2 ± 5.3 | |
| VC-X90 | 58.2 ± 9.5 | 23.5 ± 7.1 | 6.9 ± 2.1 | 0.5 ± 0.2 | n.d. | 29.0 ± 7.5 | |
| VC-X100 | 74.8 ± 8.0 | 5.6 ± 2.3 | 4.5 ± 0.9 | 0.4 ± 0.1 | n.d. | 6.9 ± 2.4 | |
| VC-CDC | 97.8 ± 0.4 | 0.2 ± 0.1 | 1.8 ± 0.5 | 0.1 ± 0.1 | n.d. | 0.2 ± 0.1 | |
| VC-500 | 1.9 ± 0.4 | 69.0 ± 3.2 | 29.1 ± 3.0 | n.d. | n.d. | 98.1 ± 6.2 | |
| VC-X70-air | 20.0 ± 2.2 | 57.9 ± 1.1 | 22.1 ± 1.2 | n.d. | n.d. | 78.2 ± 2.3 | |
| VC-X90-air | 23.3 ± 5.4 | 53.3 ± 4.2 | 22.8 ± 2.0 | n.d. | 0.6 ± 0.2 | 74.3 ± 6.2 | |
| VC-X100-air | 30.7 ± 1.7 | 48.5 ± 2.4 | 20.7 ± 1.7 | n.d. | n.d. | 67.4 ± 4.1 |
A calcination temperature of 450 °C was chosen to completely transform residual VC to vanadium oxide after initial benchmarking of the samples via thermogravimetric analysis (see the ESI, Fig. S1†). After calcination, vanadium oxide cores inside the carbon shell are clearly visible (Fig. 1D, G and J). The smallest vanadia cores are measured for VC-X100-air (d50 = 150 ± 100 nm), similar to VC-X90-air (d50 = 160 ± 110 nm), much smaller than for VC-X70-air (d50 = 410 ± 190 nm) and the calcined VC particles (VC-500 °C) (d50 = 520 ± 200 nm). As seen in Table 1, EDX measurements are in qualitative agreement with TEM and show V2O5 contents of 98.1 ± 6.2 mass% for the precursor VC particles oxidized at 500 °C and lower amounts from 78.2 ± 2.3 mass% for VC-X70-air to 67.4 ± 4.1 mass% for VC-X100-air.
To show the structure and morphology in more detail, the two step synthesis process is illustrated in Fig. 2 using energy filtered TEM (EF-TEM) based chemical mapping. As exemplified for VC, VC-X90, and VC-X90-air, we first see how VC particles were successively transformed to CDC from the outside to the inside retaining a VC core. Calcination of VC inside the composite particles leads to V2O5 domains engulfed in nanoporous VC-CDC. EF-TEM chemical mapping confirms the distribution of carbon around the V2O5. During calcination, a partial cracking of the carbon shell is induced due to expansion of the core, as a result of the different skeletal densities of 3.36 g cm−3 for V2O5 and 5.48 g cm−3 for VC.38,39
By use of Raman spectroscopy, we can track the structural changes after CDC synthesis (Fig. 3A) and calcination (Fig. 3C). It is shown that the VC signal intensity at 269 cm−1 decreases with successive transformation from VC to CDC (see the inset in Fig. 3A).40 In addition to the VC signal, the Raman spectra show incompletely graphitized carbon, with the D-mode at ca. 1340–1350 cm−1, the G-mode at ca. 1580–1600 cm−1, and a pronounced second order spectrum at 2200–3500 cm−1. The degree of carbon ordering in VC/CDC core–shell particles is significantly higher than for the fully transformed CDC by chlorine treatment (VC-CDC). This is evidenced by the broader peak shapes and transition between D- and G-mode due to amorphous carbon at ∼1520 cm−1, as well as the less distinct second order spectrum for the VC-CDC sample (Fig. 3A).41,42 However, the process stemming from NiCl2 also leads to the local emergence of metallic Ni, which, in turn, catalyzes the formation of graphitic carbon arranged in onion-like shells (Fig. 1I and J).43
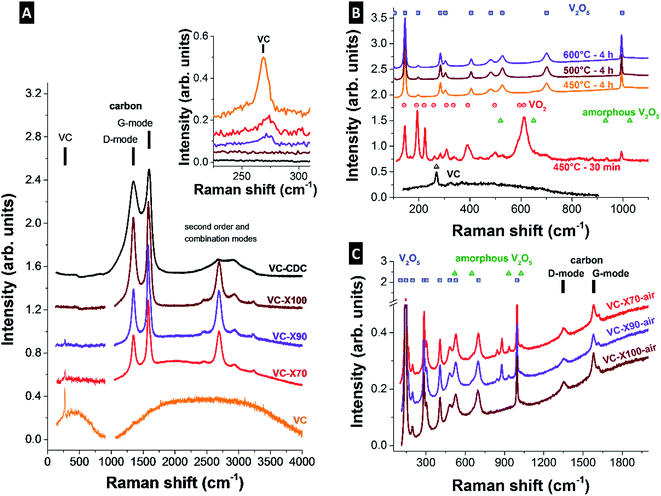 | ||
| Fig. 3 Raman spectra of (A) VC and VC/CDC core–shell particles, (B) VC calcined at different temperatures, and (C) V2O5/CDC core–shell particles after calcination at 450 °C for 30 min. The reference Raman peaks are from VC,40 VO2,46 V2O5,44 and amorphous V2O5.45 | ||
As seen from Fig. S1 (ESI†), precise control over the calcination process temperature and duration is required to avoid either incomplete carbide oxidation or complete CDC burn-off. For this reason, we conducted first oxidation experiments only on the initial VC powder. The increase in mass of the initial VC particles, without prior NiCl2 treatment, is ∼40 mass% (ESI, Fig. S1†) for an annealing at 600 °C, leading to crystalline V2O5 (Fig. 3B). However, using this calcination temperature, more than 50 mass% of the CDC was burned-off. Therefore, the synthesis was repeated at a lower temperature of 450 °C for a duration of 30 min. With this annealing process, the same mass increase (∼40 mass%) was achieved, expecting the same vanadia phase, and only ∼20 mass% of the CDC material was removed. Synthesis at 450 °C for 30 min results in a mixture of V2O5,44 amorphous V2O5,45 and VO2 (ref. 46) because of the large size of the precursor VC particles (∼1 µm) (Fig. 3B). The particles with the VC/CDC core–shell arrangement present much smaller cores than the initial VC particles (Fig. 1B), which leads to the formation of V2O5 already at 450 °C applied for 30 min (Fig. 3C). These findings are supported by X-ray diffraction (XRD), shown in the ESI, Fig. S2.† Transformation from VC to VC/CDC is clearly evidenced by the emergence of the (002) graphite signal at 26.4° 2θ for VC-X70 and the continuous increase of its intensity up to the highest degree of transformation for VC-X100 (ESI, Fig. S2A†). The fully transformed VC-CDC presents a less distinct carbon signal, coming from nanocrystalline and amorphous carbon, in agreement with Raman spectroscopy. After calcination, only V2O5 is identified (ESI, Fig. S2B†).
Highly nanoporous materials with high specific surface areas typically result from chlorine treatment of VC (i.e., VC-CDC).38 The nitrogen gas sorption isotherm of VC-CDC shows a characteristic type I(b) shape related to microporous materials with pore sizes <2.5 nm (ESI, Fig. S3A†). Both micro- and mesopores are found for the partial transformation of VC with type I(b) isotherms, related to the internal microporosity, combined with type IV(b) isotherms related to mesopores with a small H4 hysteresis (Fig. S3B†).47 The combination of micro- and mesopores has been reported in other studies on carbon core–shell structures.36 The BET surface area increases from 120 m2 g−1 for VC-X70 to 577 m2 g−1 for VC-X100, and to 1466 m2 g−1 for VC-CDC (Table 2). Calcination of VC/CDC core–shell particles does not change the pore type (micro- and mesopores), but leads to oxidation of the high surface area carbon, with consequent decrease in surface area. For VC-X100-air and VC-X90-air, the surface area collapses to 61 m2 g−1 (−89%) and 124 m2 g−1 (−38%), respectively, due to oxidation of the CDC shell. The surface area of VC-X70-air is even larger than before calcination with 310 m2 g−1 (+158%; Table 2). The high content of VC in VC-X70 (40 mass%), which is turned into the lower density V2O5, has a stronger impact on the surface area than the CDC, compared to VC-X90 and VC-X100 with lower VC contents.
| BET SSA (m2 g−1) | DFT SSA (m2 g−1) | Pore volume (cm3 g−1) | |
|---|---|---|---|
| VC-X70 | 120 | 100 | 0.185 |
| VC-X90 | 200 | 208 | 0.224 |
| VC-X100 | 577 | 485 | 0.424 |
| VC-CDC | 1466 | 1535 | 0.673 |
| VC-500 | 42 | 23 | 0.053 |
| VC-X70-air | 310 | 269 | 0.254 |
| VC-X90-air | 124 | 66 | 0.171 |
| VC-X100-air | 61 | 38 | 0.108 |
3.2 Electrochemical performance
As seen from the previous section, partial chlorination of VC to VC/CDC and successive calcination to V2O5/CDC in a facile two-step approach leads to highly crystalline V2O5 embedded graphitic and porous carbon. This combination is promising for electrochemical hybrid energy storage applications, which was benchmarked in our study in 1 M LiClO4 in ACN as the electrolyte. Cyclic voltammetry of VC-CDC using the half-cell setup shows a nearly ideal double-layer capacitor behavior with a rectangular CV shape without any redox reactions. This behavior is expected from nanoporous carbons in general and CDC materials in specific.6 VC-X70-air is characterized by double-layer capacitance on the positive side between +0.5 V and +1.2 V vs. carbon. In the negative range, a very broad redox peak emerges (Fig. 4B and ESI, Fig. S4B†). This broad peak correlates with the larger V2O5 core of VC-X70 compared to the nanotextured V2O5 particles in VC-X90-air and VC-X100-air. Due to longer ion pathways in the V2O5 crystal, the system presents slower redox reactions and broader redox peaks.In stark contrast to the capacitor-like behavior of VC-CDC, the CVs of VC-X90-air and VC-X100-air are characterized by several, very sharp redox peaks from Li-ion intercalation.30,48,49 The occurring electrochemical Li+ insertion process for V2O5 can be expressed by eqn (7):50
| V2O5 + xLi+ + xe− ↔ LixV2O5 | (7) |
Typically, a set of two peaks for inter- and deintercalation is observed between +2 and +4 V vs. Li/Li+ or −1.2 V and +0.8 V vs. carbon.48 The structural changes during Li+ intercalation into V2O5 involve several phases like α, ε, δ, and γ for x ≤ 2, with three voltage plateaus at 3.4, 3.2, and 2.3 V vs. Li/Li+ (−0.7 V, 0 V, and +0.2 V vs. carbon) (Fig. 4B).48 The plateaus seen from the galvanostatic curves (Fig. 4B) and the pairs of redox peaks in the CVs (Fig. 4A) belong to the two-phase regions α/ε, ε/δ, and δ/γ. Larger lithium contents (x > 2) for potentials below 2 V vs. Li/Li+ (−1.2 V vs. carbon) would lead to irreversible reactions and the formation of the γ-phase.48,51,52 A detailed discussion of the structural changes is already given in ref. 48 and 51–53. The first set of redox peaks emerges between −0.25 V and +0.8 V vs. carbon for inter- and deintercalation of the first Li-ion. A second lithiation step occurs between −1.2 V and 0 V vs. carbon (ESI, Fig. S4A†). For potentials above +0.8 V vs. carbon, only double-layer capacitance of the porous carbon is encountered. Calcined VC without the core–shell structure, but the same V2O5 phase, shows very broad sets of redox peaks in the CV compared to the core–shell structure. It is expected that longer ion intercalation paths of the larger size of V2O5 domains in VC-500 °C result in slower redox reactions of the electrode, similar to VC-X70-air. In contrast, the core–shell structures present much smaller V2O5 particles due to partial transformation into porous carbon.
Galvanostatic charge/discharge cycling (GCPL) in half-cells was further employed for assessing the power handling ability of the electrode materials. As seen from Fig. 4B, GCPL curves of VC-X100-air and VC-X90-air exhibited several voltage plateaus between −1.2 V and +1.2 V vs. carbon, in agreement with the redox peaks in the CVs. Nearly ideal double-layer behavior with triangular-shape GCPL curves was observed for VC-CDC. The Li+ intercalation and deintercalation leads to phase transitions during cycling and is kinetically enhanced for nanoscopic materials because of better accessibility of intercalation pathways.54 Therefore, smaller V2O5 core sizes of V2O5/CDC core–shell samples show superior rate handling compared to VC-500 with a more than 2-times larger V2O5 particle size (Fig. 4C). This is especially seen for VC-X100-air with more than 100 mA h g−1 capacity at 5 A g−1 (equivalent to a rate of ca. 28C).
The lower vanadium oxide content of VC-X100-air limits the maximum capacity at low rates. The highest value is achieved for VC-X90-air with more than 300 mA h g−1. Typically, in the literature capacity values are normalized to the active mass, in this case the mass of V2O5 in the composite electrode. Using this way of normalization, the highest capacity for VC-X90-air is 415 mA h g−1. Even for higher specific currents of 5 A g−1 the specific capacity reaches more than 100 mA h g−1 (comparable to a C-rate of 12C). The normalized values are given as an inset in Fig. 4D for comparison. Cycling in the potential window of −1.2 V to +1.2 V vs. carbon is expected to be completely reversible,48,51,52 and this behavior was confirmed for all V2O5/CDC core–shell particles with a coulombic efficiency of ca. 100% (Fig. 4D). The VC particles calcined at 500 °C with much larger particle sizes (Fig. 1B) and without CDC shells, present a lower charge efficiency of less than 80% at 20 A g−1. This might be the result of V2O5 degeneration due to kinetic limitations.30
Electrochemical properties on a device level were measured using an asymmetric full-cell setup with nanoporous VC-CDC as the counter electrode. Typically, symmetric setups for electrode materials with battery-like behavior (e.g., intercalation/deintercalation) are disadvantageous due to their polarization dependent redox-reactions. Yet, several studies used different types of V2O5 or V2O5/carbon composites, such as electrospun V2O5 nanofibers,55 V2O5/MWCNT core–shell aerogels,56 and V2O5/graphene aerogels57 with the symmetrical full-cell setup. However, in some of these studies, negative and positive polarization in the full-cell arrangement was used (e.g. −1 V to +1 V in an aqueous electrolyte), instead of 0 V to 1 V. In these cases the capacitance and specific energies are much larger reaching misleading high values of 87 W h kg−1 in aqueous 1 M Na2SO4 (ref. 56) and 78 W h kg−1 in organic 1 M LiClO4 in PC.55
Enhanced electrochemical performance can be obtained by use of an asymmetric two-electrode setup (Fig. 5). Studies so far have shown, for aqueous systems, the highest specific energy of 29 W h kg−1 (Table 3) for hydrothermally prepared V2O5·0.6H2O with activated carbon as the counter electrode.58 Due to the improved voltage stability of organic electrolytes, much higher energies can be achieved, ranging from 15 to 76 W h kg−1 with MnO2/rGO, AC fabric, or rGO as the counter electrode in an asymmetric arrangement.34,59,60 In this study, an asymmetric full-cell setup with V2O5/CDC on the negative polarization side and charge-balanced VC-CDC on the positive polarization side was used. VC-X90-air as a negative electrode material presents superior performance with a specific energy in excess of 80 W h kg−1 for charging and more than 50 W h kg−1 for discharging (Fig. 5A). Even at higher rates, with a specific power of ∼6.7 kW kg−1, the specific energy is 27 W h kg−1 (12 W h kg−1 for discharging). In agreement with half-cell measurements, VC-X100-air shows a lower specific energy of 66 W h kg−1 with comparable power handling because of the lower V2O5 content. The energy and power performance of this core–shell system is highly competitive with current literature values and is, to the best of our knowledge, the only core–shell system reaching 50–80 W h kg−1 specific energy when using asymmetric full-cell measurements and proper application of the cell voltage.61
| Material (WE) | Setup | CE | Electrolyte | Specific energy W h kg−1 | Specific power W kg−1 | Ref. |
|---|---|---|---|---|---|---|
| V2O5/CDC core–shell | asym. | CDC | 1 M LiClO4 in ACN | 84 (51) | 166 | This study |
| 27 (12) | 6653 | |||||
| V2O5/OLC | asym. | AC | 1 M LiClO4 in ACN | 38 | 57 | 33 |
| 10 | 2004 | |||||
| V2O5/CNTs | asym. | AC | 1 M LiClO4 in PC | 40 | 200 | 22 |
| 7 | 6300 | |||||
| V2O5 nanowires | asym. | AC fabric | 0.1 M LiTFSI in ACN | 46 | 662 | 59 |
| 6 | 5260 | |||||
| Li+ exchanged V2O5 NWs | asym. | AC fabric | 0.1 M LiTFSI in ACN | 66 | 720 | 59 |
| 7 | 8320 | |||||
| V2O5 NWs/rGO | asym. | MnO2/rGO | 1 M LiTFSI in ACN | 15 | 437 | 60 |
| V2O5/Pin/ACC | asym. | rGO/ACC | LiNO3/PVA gel | 39 | 900 | 65 |
| 33 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|||||
| V2O5/rGO NS | asym. | rGO | 1 M KCl | 76 | 900 | 34 |
| 25 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|||||
| V2O5·0.6H2O | asym. | AC | 0.5 M K2SO4 | 29 | 70 | 58 |
| 20 | 2000 | |||||
| Layered V2O5 aerogel | asym. | AC | 1 M Na2SO4 | 11 | 280 | 66 |
| 5 | 2200 | |||||
| V2O5/MWCNTs | sym. | WE | 1 M Na2SO4 | 87 | 250 | 56 |
| V2O5 nanofibers | sym. | WE | 1 M LiClO4 in PC | 78 | 1800 | 55 |
| 15 | 1500 | |||||
| 2 M KCl | 5 | 1700 | 55 | |||
| 3 | 1800 | |||||
| V2O5/PPy core–shell | sym. | WE | LiNO3/PVA gel | 10 | 5000 | 67 |
| V2O5/graphene | sym. | WE | 1 M Na2SO4 | 68 | 250 | 57 |
The high attractiveness of this material for electrochemical energy storage is underlined by the high cycling stability with an energy retention of more than 80% and 70% over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 galvanostatic cycles for VC-X90-air and VC-X100-air, respectively, measured at 1 A g−1 (Fig. 5B). The nearly ideal charge efficiency of the half-cell measurements (100%) translates to a still attractive energy efficiency of the asymmetric devices between 60 and 80% for low rates. For comparison, standard double-layer capacitors using activated carbon electrodes only reach slightly higher values of 80–95% dependent on the electrode.62
000 galvanostatic cycles for VC-X90-air and VC-X100-air, respectively, measured at 1 A g−1 (Fig. 5B). The nearly ideal charge efficiency of the half-cell measurements (100%) translates to a still attractive energy efficiency of the asymmetric devices between 60 and 80% for low rates. For comparison, standard double-layer capacitors using activated carbon electrodes only reach slightly higher values of 80–95% dependent on the electrode.62
4. Conclusions
This study, for the first time, demonstrates a two-step in situ synthesis of hybrid particles consisting of porous carbon and redox-active V2O5 by solely using VC as the precursor. The particles present a V2O5/CDC core–shell structure with nanoscopic V2O5 cores, completely accessible to electrolyte ions and interconnected by highly conductive carbon. High specific energies of 50 and 80 W h kg−1 for discharging and charging, respectively, were measured in a facile asymmetric full-cell setup, while simultaneously retaining ultrafast cycling. The asymmetric cell design with CDC on the positive polarization side leads to superior stability over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles and comparably high energy efficiency of 60–80% at low rates. The novel synthesis procedure can easily be scaled up by means of larger precursor mass and may also be very attractive for other redox-active compounds.
000 cycles and comparably high energy efficiency of 60–80% at low rates. The novel synthesis procedure can easily be scaled up by means of larger precursor mass and may also be very attractive for other redox-active compounds.
Acknowledgements
M. Z., B. K., and V. P. gratefully acknowledge funding from the German Federal Ministry for Research and Education (BMBF) in support of the nanoEES3D project (award number 03EK3013) as part of the strategic funding initiative energy storage framework and Prof. Eduard Arzt (INM) for his continuing support. T. A., and B. E. gratefully acknowledge the funding of the German Federal Ministry of Education and Research (BMBF) under the project AktivCAPs (award number 02E2-ESP077) and the German Research Council (DFG), which, within the framework of its “Excellence Initiative”, supports the Cluster of Excellence “Engineering of Advanced Materials” (http://www.eam.uni-erlangen.de) at the University of Erlangen-Nuremberg. T. A. gratefully acknowledges the Indonesian Directorate General of Higher Education (DIKTI) for a PhD scholarship. Dr Mesut Aslan is thanked for vanadium carbide chlorination.References
- P. Simon, Y. Gogotsi and B. Dunn, Science, 2014, 343, 1210–1211 CrossRef CAS PubMed
.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed
.
- J. B. Goodenough, Energy Environ. Sci., 2014, 7, 14–18 CAS
.
- Z. Yang, J. Zhang, M. C. Kintner-Meyer, X. Lu, D. Choi, J. P. Lemmon and J. Liu, Chem. Rev., 2011, 111, 3577–3613 CrossRef CAS PubMed
.
- J. R. Miller, J. Power Sources, 2016, 326, 726–735 CrossRef CAS
.
- F. Béguin, V. Presser, A. Balducci and E. Frackowiak, Adv. Mater., 2014, 26, 2219–2251 CrossRef PubMed
.
- N. Jäckel, M. Rodner, A. Schreiber, J. Jeongwook, M. Zeiger, M. Aslan, D. Weingarth and V. Presser, J. Power Sources, 2016, 326, 660–671 CrossRef
.
- V. Presser, M. Heon and Y. Gogotsi, Adv. Funct. Mater., 2011, 21, 810–833 CrossRef CAS
.
- O. Barbieri, M. Hahn, A. Herzog and R. Kötz, Carbon, 2005, 43, 1303–1310 CrossRef CAS
.
- J. Chmiola, G. Yushin, Y. Gogotsi, C. Portet, P. Simon and P. L. Taberna, Science, 2006, 313, 1760–1763 CrossRef CAS PubMed
.
- E. Frackowiak, K. Fic, M. Meller and G. Lota, ChemSusChem, 2012, 5, 1181–1185 CrossRef CAS PubMed
.
- B. E. Conway, V. Birss and J. Wojtowicz, J. Power Sources, 1997, 66, 1–14 CrossRef CAS
.
- M. Salanne, B. Rotenberg, K. Naoi, K. Kaneko, P.-L. Taberna, C. Grey, B. Dunn and P. Simon, Nat. Energy, 2016, 1, 16070 CrossRef
.
- J. Lee, B. Krüner, A. Tolosa, S. Sethuraman, D. Kim, S. Choudhury, K. H. Seo and V. Presser, Energy Environ. Sci., 2016, 9, 3392–3398 CAS
.
- T. Brousse, D. Bélanger and J. W. Long, J. Electrochem. Soc., 2015, 162, A5185–A5189 CrossRef CAS
.
- J. Zheng, P. Cygan and T. Jow, J. Electrochem. Soc., 1995, 142, 2699–2703 CrossRef CAS
.
- C. Xu, F. Kang, B. Li and H. Du, J. Mater. Res., 2010, 25, 1421–1432 CrossRef CAS
.
- M. Ghidiu, M. R. Lukatskaya, M.-Q. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2014, 516, 78–81 CAS
.
- S. Roldán, C. Blanco, M. Granda, R. Menéndez and R. Santamaría, Angew. Chem., Int. Ed., 2011, 50, 1699–1701 CrossRef PubMed
.
- M. Zeiger, D. Weingarth and V. Presser, ChemElectroChem, 2015, 2, 1117–1127 CrossRef CAS
.
- N. Nitta, F. Wu, J. T. Lee and G. Yushin, Mater. Today, 2015, 18, 252–264 CrossRef CAS
.
- Z. Chen, V. Augustyn, J. Wen, Y. Zhang, M. Shen, B. Dunn and Y. Lu, Adv. Mater., 2011, 23, 791–795 CrossRef CAS PubMed
.
- J. S. Daubert, N. P. Lewis, H. N. Gotsch, J. Z. Mundy, D. N. Monroe, E. C. Dickey, M. D. Losego and G. N. Parsons, Chem. Mater., 2015, 27, 6524–6534 CrossRef CAS
.
- L.-M. Chen, Q.-Y. Lai, Y.-J. Hao, Y. Zhao and X.-Y. Ji, J. Alloys Compd., 2009, 467, 465–471 CrossRef CAS
.
- T. Kudo, Y. Ikeda, T. Watanabe, M. Hibino, M. Miyayama, H. Abe and K. Kajita, Solid State Ionics, 2002, 152–153, 833–841 CrossRef CAS
.
- D. Chao, X. Xia, J. Liu, Z. Fan, C. F. Ng, J. Lin, H. Zhang, Z. X. Shen and H. J. Fan, Adv. Mater., 2014, 26, 5794–5800 CrossRef CAS PubMed
.
- Y. L. Cheah, V. Aravindan and S. Madhavi, ACS Appl. Mater. Interfaces, 2013, 5, 3475–3480 CAS
.
- D. Zhu, H. Liu, L. Lv, Y. D. Yao and W. Z. Yang, Scr. Mater., 2008, 59, 642–645 CrossRef CAS
.
- M. Sathiya, A. S. Prakash, K. Ramesha, J. M. Tarascon and A. K. Shukla, J. Am. Chem. Soc., 2011, 133, 16291–16299 CrossRef CAS PubMed
.
- Y. Liu, M. Clark, Q. Zhang, D. Yu, D. Liu, J. Liu and G. Cao, Adv. Energy Mater., 2011, 1, 194–202 CrossRef CAS
.
- M.-S. Balogun, Y. Luo, F. Lyu, F. Wang, H. Yang, H. Li, C. Liang, M. Huang, Y. Huang and Y. Tong, ACS Appl. Mater. Interfaces, 2016, 8, 9733–9744 CAS
.
- Q. Xu and T. Zhao, Prog. Energy Combust. Sci., 2015, 49, 40–58 CrossRef
.
- S. Fleischmann, N. Jäckel, M. Zeiger, B. Krüner, I. Grobelsek, P. Formanek, S. Choudhury, D. Weingarth and V. Presser, Chem. Mater., 2016, 28, 2082–2813 CrossRef
.
- D. H. Nagaraju, Q. Wang, P. Beaujuge and H. N. Alshareef, J. Mater. Chem. A, 2014, 2, 17146–17152 CAS
.
- M. Schmirler, F. Glenk and B. J. Etzold, Carbon, 2011, 49, 3679–3686 CrossRef CAS
.
- T. Ariyanto, B. Dyatkin, G.-R. Zhang, A. Kern, Y. Gogotsi and B. J. Etzold, Microporous Mesoporous Mater., 2015, 218, 130–136 CrossRef CAS
.
- C. R. Pérez, S. H. Yeon, J. Ségalini, V. Presser, P. L. Taberna, P. Simon and Y. Gogotsi, Adv. Funct. Mater., 2013, 23, 1081–1089 CrossRef
.
- A. Jänes, T. Thomberg and E. Lust, Carbon, 2007, 45, 2717–2722 CrossRef
.
-
J. G. Speight, Lange's Handbook of Chemistry, McGraw-Hill, New York, 16 edn, 2005 Search PubMed
.
- T. Huang, S. Mao, G. Zhou, Z. Wen, X. Huang, S. Ci and J. Chen, Nanoscale, 2014, 6, 9608–9613 RSC
.
- A. Ferrari and J. Robertson, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 14095 CrossRef CAS
.
- A. C. Ferrari and J. Robertson, Phys. Rev. B, 2001, 64, 075414 CrossRef
.
- Z. Huang, D. Wang, J. Wen, M. Sennett, H. Gibson and Z. Ren, Appl. Phys. A: Mater. Sci. Process., 2002, 74, 387–391 CrossRef CAS
.
- R. Baddour-Hadjean, J. P. Pereira-Ramos, C. Navone and M. Smirnov, Chem. Mater., 2008, 20, 1916–1923 CrossRef CAS
.
- S.-H. Lee, H. M. Cheong, M. J. Seong, P. Liu, C. E. Tracy, A. Mascarenhas, J. R. Pitts and S. K. Deb, Solid State Ionics, 2003, 165, 111–116 CrossRef CAS
.
- G. Petrov, V. Yakovlev and J. Squier, Appl. Phys. Lett., 2002, 81, 1023–1025 CrossRef CAS
.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. Sing, Pure Appl. Chem., 2015, 87, 1051–1069 CrossRef CAS
.
- C. Leger, S. Bach, P. Soudan and J.-P. Pereira-Ramos, J. Electrochem. Soc., 2005, 152, A236–A241 CrossRef CAS
.
- M. Koltypin, V. Pol, A. Gedanken and D. Aurbach, J. Electrochem. Soc., 2007, 154, A605–A613 CrossRef CAS
.
- J. Livage, Chem. Mater., 1991, 3, 578–593 CrossRef CAS
.
- C. Delmas, H. Cognac-Auradou, J. Cocciantelli, M. Menetrier and J. Doumerc, Solid State Ionics, 1994, 69, 257–264 CrossRef CAS
.
- C. Delmas, S. Brethes and M. Menetrier, J. Power Sources, 1991, 34, 113–118 CrossRef CAS
.
- M. S. Whittingham, Chem. Rev., 2004, 104, 4271–4302 CrossRef CAS PubMed
.
- V. Augustyn, P. Simon and B. Dunn, Energy Environ. Sci., 2014, 7, 1597–1614 CAS
.
- G. Wee, H. Z. Soh, Y. L. Cheah, S. G. Mhaisalkar and M. Srinivasan, J. Mater. Chem., 2010, 20, 6720–6725 RSC
.
- Y. Wu, G. Gao, H. Yang, W. Bi, X. Liang, Y. Zhang, G. Zhang and G. Wu, J. Mater. Chem. A, 2015, 3, 15692–15699 CAS
.
- Y. Wu, G. Gao and G. Wu, J. Mater. Chem. A, 2015, 3, 1828–1832 CAS
.
- Q. T. Qu, Y. Shi, L. L. Li, W. L. Guo, Y. P. Wu, H. P. Zhang, S. Y. Guan and R. Holze, Electrochem. Commun., 2009, 11, 1325–1328 CrossRef CAS
.
- S. D. Perera, B. Patel, N. Nijem, K. Roodenko, O. Seitz, J. P. Ferraris, Y. J. Chabal and K. J. Balkus, Adv. Energy Mater., 2011, 1, 936–945 CrossRef CAS
.
- S. D. Perera, M. Rudolph, R. G. Mariano, N. Nijem, J. P. Ferraris, Y. J. Chabal and K. J. Balkus Jr, Nano Energy, 2013, 2, 966–975 CrossRef CAS
.
- M. D. Stoller and R. S. Ruoff, Energy Environ. Sci., 2010, 3, 1294–1301 CAS
.
- A. Laheäär, P. Przygocki, Q. Abbas and F. Béguin, Electrochem. Commun., 2015, 60, 21–25 CrossRef
.
- T. Ariyanto, A. M. Laziz, J. Gläsel, G.-R. Zhang, J. Garbes and B. J. Etzold, Chem. Eng. J., 2016, 283, 676–681 CrossRef CAS
.
- S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 60, 11 CrossRef
.
- X. Zhou, Q. Chen, A. Wang, J. Xu, S. Wu and J. Shen, ACS Appl. Mater. Interfaces, 2016, 8, 3776–3783 CAS
.
- C. X. Guo, G. Yilmaz, S. Chen, S. Chen and X. Lu, Nano Energy, 2015, 12, 76–87 CrossRef CAS
.
- T. Qian, N. Xu, J. Zhou, T. Yang, X. Liu, X. Shen, J. Liang and C. Yan, J. Mater. Chem. A, 2015, 3, 488–493 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ta08900c |
| This journal is © The Royal Society of Chemistry 2016 |