Open Access Article
Open Access ArticleSynthesis of modified fullerenes for oxygen reduction reactions†
Rosa
María Girón
a,
Juan
Marco-Martínez
a,
Sebastiano
Bellani
bc,
Alberto
Insuasty
a,
Hansel
Comas Rojas
bd,
Gabriele
Tullii
b,
Maria Rosa
Antognazza
*b,
Salvatore
Filippone
*a and
Nazario
Martín
*ae
aDepartamento de Química Orgánica I, Facultad de Ciencias Químicas, Universidad Complutense de Madrid, Ciudad Universitaria s/n, 28040 Madrid, Spain. E-mail: salvatorefilippone@ucm.es; nazmar@quim.ucm.es
bCenter for Nano Science and Technology@PoliMi, Istituto Italiano di Tecnologia, Via Pascoli 70/3, 20133 Milano, Italy. E-mail: mariarosa.antognazza@iit.it
cIstituto Italiano di Tecnologia, Graphene Labs, Via Morego 30, Genova 16163, Italy
dHigher Institute for Applied Sciences and Technologies (INSTEC), Salvador Allende y Luaces, 6163, La Habana, Cuba
eIMDEA-Nanociencia, Campus de la Universidad Autónoma de Madrid, 28049 Madrid, Spain
First published on 15th August 2016
Abstract
The oxygen reduction reaction (ORR) is a key process common in several energy converting systems or electro-chemical technologies such as fuel cells, metal–air batteries, oxygen sensors, etc., which is based on the use of expensive and scarcely available platinum metal. In the search for carbon-based catalysts for ORRs, two different classes of new fullerene hybrids and metal-free fullerene derivatives endowed with suitable active sites have been prepared by highly selective metal- and organo-catalyzed synthetic methodologies. Along with their classical behavior as electron acceptors in polymer-based photo-electrochemical cells, the new fullerene derivatives are able to efficiently catalyze ORRs by using no metals or very low amounts of metals. Remarkably, the activity of metal-free fullerenes has proved to be as high as that observed for metallofullerenes bearing noble metals, and up to ten-fold higher than that of PCBM.
1 Introduction
The oxygen reduction reaction (ORR) is a key process in different energy converting systems or electrochemical technologies (fuel cells, metal air batteries, oxygen sensors, etc.).1 In these fields the replacement of the traditional platinum based catalysts with non-precious metals2 or metal-free electrocatalysts is currently a hot scientific challenge.3 Particularly, the use of carbon nanomaterials such as nitrogen-doped carbon nanotubes4 or graphene,5 represents an interesting and novel approach.In this context, among the different allotropic forms of carbon, fullerenes combine unique chemical,6 optical,7 electronic8 and photophysical9 properties with a defined molecular structure. Indeed, their good electron accepting and transport capability led to their extended use as suitable n-type materials,10 widely employed in organic electronics and photovoltaic devices,11 including the most recent perovskite solar cells.12
Thus, bulk heterojunction (BHJ) organic photovoltaic cells (OPVs) are a notable application, which consist of p-type conjugated polymers (e.g. poly(3-hexylthiophene-2,5-diyl), P3HT) and n-type fullerene derivatives, [6,6]phenyl-C61-butyric acid methyl ester (PCBM) being the most widely used. In a typical BHJ approach, a bi-continuous network is created, which allows the promotion of exciton dissociation processes and the achievement of a high power conversion efficiency. The next frontier in the use of acceptor/donor BHJs, based on PCBM, is the realization of photo-electrochemical cells, able to work in contact with aqueous and/or non-aqueous electrolytes upon visible light illumination. One notable example is the realization of efficient photocathodes for hydrogen production by photo-electrochemical water splitting.13,14 Recently, some of us have reported a high sensitivity, photo-electrochemical oxygen sensor based on an organic BHJ formed using a low band gap polymer (APFO-3) and PCBM, revealing photo-activity towards the ORR.15 In the proposed scheme, polymer excitation by visible light leads to the efficient generation of bounded charged species (excitons), which are promptly dissociated into free charges (polarons) by a highly efficient electron transfer process to the fullerene-based acceptor domain. We have recently reported that this process, typical of solid-state photovoltaic cells, occurs in the hybrid system with fully comparable dynamics and efficiency.16 Photogenerated holes are collected at the underlying fluorine-doped tin oxide (FTO) electrode, while electrons are efficiently transferred at the interface with the aqueous solution, thus giving rise to photoelectrochemical reactions and, in particular, to the ORR (Fig. 1).
 | ||
| Fig. 1 Schematic sketch of an organic-based photo-electrochemical device for the ORR, based on P3HT:fullerene BHJ thin films. The working mechanism and the energy level schemes are also represented. | ||
Here, we report the synthesis of new fullerene derivatives, suitably functionalized with catalytically active sites towards the ORR. This allows us to exploit a doubled fullerene functionality, by employing them both as electron acceptors in P3HT-based BHJ, and as redox active sites, thus avoiding the use of expensive noble metals.
Two main approaches have been undertaken in parallel: (i) the synthesis of new fullerene hybrid derivatives endowed with noble metals active in redox processes, such as Ir, Rh or Pt; (ii) the synthesis of metal-free fullerene catalysts, based on the presence of active C60–H bonds. To this aim, both metal- and organo-catalytic methodologies have been employed, to obtain fullerene derivatives with tailored electronic and photocatalytic properties characterized by high regio- and stereo-selectivity.
Photoelectrochemical devices for the ORR based on BHJ P3HT:fullerene thin films have been finally realized, confirming an enhanced photocatalytic property of the novel compounds, when compared with the extensively used PCBM, taken as the reference fullerene.
2 Results and discussion
2.1. Synthesis of metallo- and organo-fullerenes catalysts
Over the last few years, fullerene chemistry has been widening its synthetic tools, in the search for more sophisticated structures,17 for a better control on the stereochemistry of the fullerene derivatives18 and for a control on the regio- and site-selectivity when higher fullerenes19 or endohedral fullerenes20 are used. Following this trend, on one hand, we have directed our attention to the achievement of new selective catalytic methodologies aimed at the preparation of both metallo-fullerenes and regiopure fullerene hydrides. On the other hand, we have modified both metal- and organo-catalysed methodologies,21 previously described by us, for the introduction of a suitable functionality on the fullerene cage to become catalytically active towards the ORR. | ||
| Scheme 1 Diastereomeric synthesis of iridium (2Ir) and rhodium (2Rh) pyrrolino[3,4:1,2][60]fullerene. | ||
Indeed, this behaviour was observed by 1H-NMR (see the ESI†) and its structure could be confirmed by X-ray diffraction analysis of a monocrystal obtained by slow evaporation of 2Ir in CS2/hexane.24
As is shown in Fig. 2, the iridium atom adopts a pseudo-octahedral geometry where the Cp* group occupies a face of the octahedron, 1.775 Å being the distance between the metal and the ring centroid. Two other octahedral positions involve the chelate pyrrolinofullerene carboxylate featuring Ir–N and Ir–O bonds of 2.103(4) Å and 2.095(4) Å length, respectively. Finally, the determined Ir–Cl bond distance resulted to be 2.393(2) Å, which is slightly shorter than that of other related pyrrolidinocarboxylate iridium Cp* complexes.23b The distance between the two fullerene sp3 carbon atoms is 1.590(8) Å, which is in the typical range for a fullerene monoadduct.
X-ray analysis confirms the presence of a single diastereomer (with both enantiomers) with the chlorine atom in a trans position to the methyl group of the iridium cycle (see also ESI†).
Analogously, a rhodium pyrroline[60]fullerene hybrid 2Rh was prepared in 38% yield, by using [Cp*RhCl2]2 after the cycloaddition of the same azlactone 1 (Scheme 1).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds have been envisaged as very useful molecular species for hydrogen storage and, therefore, fullerene hydrides (or fulleranes) have been deeply studied.25,26 Furthermore, these species easily undergo dehydrogenation in the presence of even very low amount of molecular oxygen.27 In this regard, we wondered if the presence of highly active hydrogen directly linked to the carbon cage could replace precious metals but still maintain a high catalytic activity. Thus, we designed a regioselective double addition on [60]fullerene where a C60–H bond is formed by the use of the organocatalytic methodology. To this aim, we extended the scope of our previously reported phosphine catalyzed cycloaddition of allenoates/alkynoates to [60]fullerene21b,28 to 5-hydroxy-3-alkynoates (Scheme 2).
C double bonds have been envisaged as very useful molecular species for hydrogen storage and, therefore, fullerene hydrides (or fulleranes) have been deeply studied.25,26 Furthermore, these species easily undergo dehydrogenation in the presence of even very low amount of molecular oxygen.27 In this regard, we wondered if the presence of highly active hydrogen directly linked to the carbon cage could replace precious metals but still maintain a high catalytic activity. Thus, we designed a regioselective double addition on [60]fullerene where a C60–H bond is formed by the use of the organocatalytic methodology. To this aim, we extended the scope of our previously reported phosphine catalyzed cycloaddition of allenoates/alkynoates to [60]fullerene21b,28 to 5-hydroxy-3-alkynoates (Scheme 2).
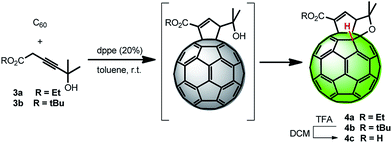
Thus, after alkynoate/allenoate isomerization, 1,2-diphenylphosphino ethane (dppe) catalysed Lu's [3 + 2] cycloaddition29 of 3a, b afforded the corresponding cyclopentenoate[60]fullerenes. These products are not isolated since they underwent an easy regioselective addition of the hydroxyl group to the cis-1 double bond of C60 giving rise to bisadducts 4a, b endowed with a C60–H bond in 48% and 34% yields, respectively. Eventually, the corresponding acid 4c was obtained after acidic removal of the tert-butyl ester.
2.2. Photocatalytic activity of fullerene derivatives toward the ORR in photoelectrochemical devices
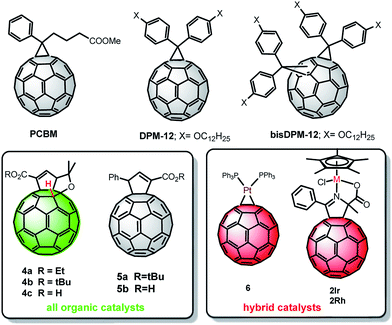
The new fullerene derivatives were finally tested as photo-electrocatalysts in polymer-based devices for the photo-electrochemical ORR, and the overall electrochemical performances were compared to those of devices employing PCBM as a standard reference material. The compounds used are classified in Chart 1 into three main types: fullerenes without catalytic sites, but with higher-lying LUMO levels compared to PCBM, as mere acceptor components (DPM-12 and bisDPM-12, in grey colour, top panel); hybrid fullerenes with metallic catalytic sites (2Ir, 2Rh and 6 compounds, in red) and catalytic, metal-free fullerenes (4a, 4b and 4c compounds, in green).BHJ thin films (∼140 nm), based on rr-P3HT as a photoactive donor component, were deposited by spin coating on top of FTO-covered glass substrates. In some cases, a second thin film fullerene layer was deposited on top of the photoactive component, in order to directly expose the catalytic sites to the electrolyte. Photo-catalytic activity towards oxygen reduction was assessed in sodium phosphate buffer (PBS) at pH 7.4 and a controlled dissolved oxygen (DO) concentration (5.8 mg L−1). All the details about material absorbance spectra, device fabrication processing, experimental set up and measurement conditions are reported in the ESI section.†
Fig. 3 reports Linear Scan Voltammetry (LSV) recorded in the dark and upon illumination (1 sun) in devices based on fullerene acceptors without any catalytic site (PCBM, DPM-12 and bisDPM-12).
As demonstrated in a previous work,15 the recorded current signal can be unambiguously attributed to photo-activated electrochemical reactions occurring at the hybrid organic/electrolyte interface and in particular to the ORR (see also Fig. S2 in the ESI section†). Moreover, dark current values (dashed lines) are negligible in all cases.
Notably, the use of DPM-12 and bisDPM-12 fullerene bisadducts allows the increase of the Onset Potential (OP, defined here as the voltage to which the photocurrent density amounts at 100 nA cm−2) up to 0.15 V vs. Ag/AgCl and 0.35 V vs. Ag/AgCl, respectively, with respect to the reference PCBM, 0.12 V vs. Ag/AgCl. Correspondingly, at a fixed voltage the photocurrent density increases: at −0.15 V vs. Ag/AgCl, for instance, it amounts to −2.4 μA cm−2, −5.2 μA cm−2, and −8.2 μA cm−2 for PCBM, DPM-12 and bisDPM-12, respectively. An analogous behavior has also been reported in organic solar cells, where an increase of the open circuit voltage in the case of DPM-12 and bisDPM-12 was reported, when compared to PCBM.30 The enhanced performances are possibly attributed to the broader density of states and to the higher-lying LUMO level of the fullerene bisadducts, able to facilitate the electron transfer processes occurring at the organic/electrolyte interfaces.
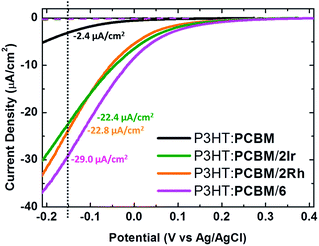
Iridium, rhodium and platinum are known to be efficient catalysts for the ORR.31 Hybrid catalysts, 2Ir, 2Rh and 6, are thus expected to provide catalytic properties typical of the embedded metal component, but with a consistently reduced need for precious metals, and with the advantage of a more localized interaction. In this case photo-electrodes were realized by covering the reference P3HT:PCBM BHJ layer with an over-layer of pristine electron acceptor, in order to maximize the localization at the electrolyte interface of the catalytic sites present within the functionalized fullerene derivatives (P3HT:PCBM/hybrid catalyst configuration).
The adopted strategy resulted to be successful. All tested hybrid catalysts show photocurrent density values larger by more than one order of magnitude with respect to reference PCBM, and comparable dark current values (Fig. 4). Photocurrent generation is clearly related to the presence of dissolved oxygen, and its origin can be safely attributed to the occurrence of the ORR (see ESI, Fig. S4,† panels a and b). OPs are also considerably increased, shifting from 0.12 V vs. Ag/AgCl for PCBM up to 0.3 V vs. Ag/AgCl for 2Ir and 2Rh and to 0.35 V vs. Ag/AgCl for 6. The reported data allow us to conclude that electrons can be easily transferred towards metallic catalytic centres which, in turn are highly efficient in reducing the overpotential needed to foster the ORR. In other words, the use of hybrid fullerenes endowed with catalytic centres increases both the electron transfer rate (observed as a net photocurrent density increase) and the driving force for electrochemical reactions (observed as an OP increase).
On the other hand, following the recent trend of using carbon based materials as the catalyst for the ORR,3–5 we planned the preparation of a molecular organocatalyst by replacement of the metallic atoms from the fullerene derivatives, with a fullerene hydride as the active site. The choice of using catalysts such as 4a–c relies on the reported hydrogen transfer from the fullerene hydrides, namely C60H2, to dioxygen27 and on the easy deprotonation of fullerene hydrides leading to very stable fullerene anions 4−.26
In order to evaluate the importance of the hydrogen fullerene bond, devices based on analogous cyclopentenoate functionalization but lacking the C60–H bond (5a, b) were also prepared.
Fig. 5 summarizes the results of the characterization of photo-electrochemical cells based on 4a, 4b, 4c, 5a and 5b components as hybrid electron acceptor/catalyst components, as compared to the reference device based on PCBM.
We notice that 4a leads to higher catalytic activity, with a more than 5-fold increase in photocurrent density at −0.15 V vs. Ag/AgCl with respect to PCBM, and a higher OP value (0.3 V vs. Ag/AgCl). Conversely, 5a and 5b, despite presenting a similar cyclopentenoate moiety on the C60 cage, do not show a significant increase of the performance due to the lack of the C60–H bond. While 5a exhibits a similar behaviour to PCBM, the higher hydrophilicity of 5b, bearing a carboxylic acid group, leads to a closer interaction with dissolved oxygen molecules, which could explain the higher photocurrent densities observed in 5b.
Finally, we further functionalized 5a and 5b compounds by directly linking the active hydrogen to the carbon cage, as in the case of the more efficient 4a compound, obtaining 4b and 4c, respectively. Once more, photocurrent generation is related to the occurrence of the ORR (see ESI, Fig. S4, panel c†). Additional measurements under O2-saturated conditions on 4b and 4c are shown in the ESI (Fig. S5†). These data have been compared with those based on PCBM. Importantly, the recorded photocurrent densities for 4b and 4c show values comparable to the ones obtained for the hybrid catalysts P3HT:PCBM/2Ir and P3HT:PCBM/2Rh, in the order of −20 μA cm−2 at −0.15 V vs. Ag/AgCl. OPs are 0.28 V in both cases which are lower with respect to the ones of precious metal hybrid samples (0.3 V vs. Ag/AgCl for PCBM/2Ir and 0.35 V vs. Ag/AgCl for PCBM/2Rh and PCBM/6) suggesting the need for further improvement. Conversely, metal-free fullerene samples showed higher durability with respect to the precious metal hybrid samples. As a figure of merit, the photocurrents recorded at −0.2 V vs. Ag/AgCl for consecutive Cyclic Voltammetry (CV) cycles have been taken (see ESI, Fig. S6†). At the fortieth cycle a decrease of the photocurrent of 12.9%, 21.2% and 45.4%, with respect to the value recorded at the first cycle, is observed for the case of PCBM, 4c and 6, respectively. Interestingly, while for PCBM and 4c the photocurrent reached stable values after a few cycles of stabilization, in the case of 6 a progressive decrease is observed. This could be due to different ORR mechanisms for the hybrid catalyst when compared to the other cases. As a matter of fact, for the Pt electrode, two-electron reduction of oxygen to H2O2 occurs parallel to the four-electron reduction to H2O (see ESI, Scheme S1†). Consequently, a decrease of the cell voltage could arise because of the lower reversible redox potential of H2O2. Similar effects have been also reported in the durability studies of fuel cells.32
Regarding the catalytic activity of the metal-free fullerene derivatives 4a–c, a plausible mechanism to account for the photocurrent increase and observed stability is based on the initial transfer of the photo-generated electron (PET) from the polymer to catalysts 4a–c affording the corresponding fullerene radical anion (Scheme 3). Thus, an easier oxygen reduction takes place to form the hydroperoxyl radical, through a hydrogen atom transfer (HAT) or by a proton coupled electron transfer (PCET), driven by the formation of the highly stable fullerene anions 4−.
The aforementioned results show the remarkable activity of fullerene-based multiple metal-free catalysts in ORR, with efficiencies comparable to those obtained from metal-containing hybrid catalysts.
3 Conclusions
In summary, we have reported the highly selective catalytic synthesis of two series of fullerene-based molecular catalysts for the ORR. Iridium and rhodium pyrrolinofullerene complexes were prepared with complete diastereoselectivity (d.e. > 99%) and the structure of the iridium–fullerene complex was confirmed by X-ray analysis. On the other hand, metal-free fullerene catalysts, endowed with a highly active C60–H bond, have been obtained by a regioselective cis-1 addition to the C60 cage. The electrocatalytic activity versus the oxygen reduction reactions has been tested in bulk heterojunction photo-electrochemical cells, affording current values up to ten fold higher than those of widely used PCBM. Remarkably, metal-free fullerene derivatives proved to give photocurrents comparable to those of related hybrids, thanks to the highly active C60–H bond on the fullerene cage. Having demonstrated that the approach is highly promising, future efforts will focus on further improvement of the overall performances of photoelectrochemical cells.Acknowledgements
We acknowledge the financial support of the European Community through the Future and Emerging Technologies (FET) programme under the FP7, collaborative Project contract no. 309223 (PHOCS). NM thanks the European Research Council ERC-2012-ADG_20120216 (Chirallcarbon), MINECO of Spain (CTQ2014-52045-R) and the CAM (FOTOCARBON project S2013/MIT-2841). R. M. G. thanks Ministerio de Educación, Cultura y Deporte for the FPU contract.Notes and references
- (a) B. C. H. Steele and A. Heinzel, Nature, 2001, 414, 345–352 CrossRef CAS PubMed; (b) F. Cheng and J. Chen, Chem. Soc. Rev., 2012, 41, 2172–2192 RSC.
- Z. Chen, D. Higgins, A. Yu, L. Zhang and J. Zhang, Energy Environ. Sci., 2011, 4, 3167–3192 CAS.
- (a) L. Dai, Y. Xue, L. Qu, H.-J. Choi and J.-B. Baek, Chem. Rev., 2015, 115, 4823–4892 CrossRef CAS PubMed; (b) D.-W. Wang and D. Su, Energy Environ. Sci., 2014, 7, 576–591 RSC.
- K. Gong, F. Du, Z. Xia, M. Durstock and L. Dai, Science, 2009, 323, 760–764 CrossRef CAS PubMed.
- (a) D. Higgins, P. Zamani, A. Yu and Z. Chen, Energy Environ. Sci., 2016, 9, 357–390 RSC; (b) J. Guan, X. Chen, T. Wei, F. Liu, S. Wang, Q. Yang, Y. Lu and S. Yang, J. Mater. Chem. A, 2015, 3, 4139–4146 RSC.
- A. Hirsch and M. Brettreich, Fullerenes: Chemistry and Reactions, Wiley VCH, Weinheim, Germany, 2005 Search PubMed.
- L. W. Tutt and A. Kost, Nature, 1992, 356, 225–226 CrossRef CAS.
- L. Echegoyen and L. E. Echegoyen, Acc. Chem. Res., 1998, 31, 593–601 CrossRef CAS.
- D. M. Guldi and M. Prato, Acc. Chem. Res., 2000, 33, 695–703 CrossRef CAS PubMed.
- J. E. Anthony, A. Facchetti, M. Heeney, S. R. Marder and X. Zhan, Adv. Mater., 2010, 22, 3876–3892 CrossRef CAS PubMed.
- (a) J. L. Delgado, P.-A. Bouit, S. Filippone, M. A. Herranz and N. Martin, Chem. Commun., 2010, 46, 4853–4865 RSC; (b) B. C. Thompson and J. M. J. Fréchet, Angew. Chem., Int. Ed., 2008, 47, 58–77 CrossRef CAS PubMed.
- (a) A. Abrusci, S. D. Stranks, P. Docampo, H.-L. Yip, A. K. Y. Jen and H. J. Snaith, Nano Lett., 2013, 13, 3124–3128 CrossRef CAS PubMed; (b) P.-W. Liang, C.-C. Chueh, S. T. Williams and A. K. Y. Jen, Adv. Energy Mater., 2015, 5, 1402321–1402328 CrossRef.
- (a) F. Fumagalli, S. Bellani, M. Schreier, S. Leonardi, H. C. Rojas, A. Ghadirzadeh, G. Tullii, A. Savoini, G. Marra, L. Meda, M. Gratzel, G. Lanzani, M. T. Mayer, M. R. Antognazza and F. Di Fonzo, J. Mater. Chem. A, 2016, 4, 2178–2187 RSC; (b) T. Bourgeteau, D. Tondelier, B. Geffroy, R. Brisse, R. Cornut, V. Artero and B. Jousselme, ACS Appl. Mater. Interfaces, 2015, 7, 16395–16403 CrossRef CAS PubMed; (c) M. Haro, C. Solis, G. Molina, L. Otero, J. Bisquert, S. Gimenez and A. Guerrero, J. Phys. Chem. C, 2015, 119, 6488–6494 CrossRef CAS; (d) G. M. Suppes, P. J. Fortin and S. Holdcroft, J. Electrochem. Soc., 2015, 162, H551–H556 CrossRef CAS; (e) M. P. Gustafson, N. Clark, B. Winther-Jensen and D. R. MacFarlane, Electrochim. Acta, 2014, 140, 309–313 CrossRef CAS.
- H. Comas Rojas, S. Bellani, F. Fumagalli, G. Tullii, S. Leonardi, M. T. Mayer, M. Schreier, M. Grätzel, G. Lanzani, F. Di Fonzo and M. R. Antognazza, Energy Environ. Sci., 2016 10.1039/c6ee01655c.
- S. Bellani, A. Ghadirzadeh, L. Meda, A. Savoini, A. Tacca, G. Marra, R. Meira, J. Morgado, F. Di Fonzo and M. R. Antognazza, Adv. Funct. Mater., 2015, 25, 4531–4538 CrossRef CAS.
- A. Guerrero, M. Haro, S. Bellani, M. R. Antognazza, L. Meda, S. Gimenez and J. Bisquert, Energy Environ. Sci., 2014, 7, 3666–3673 CAS.
- N. Martín, M. Altable, S. Filippone and A. Martín-Domenech, Synlett, 2007, 3077 CrossRef.
- E. E. Maroto, M. Izquierdo, S. Reboredo, J. Marco-Martínez, S. Filippone and N. Martín, Acc. Chem. Res., 2014, 47, 2660–2670 CrossRef CAS PubMed.
- E. E. Maroto, A. de Cózar, S. Filippone, Á. Martín-Domenech, M. Suarez, F. P. Cossío and N. Martín, Angew. Chem., Int. Ed., 2011, 50, 6060–6064 CrossRef CAS PubMed.
- (a) E. E. Maroto, J. Mateos, M. Garcia-Borràs, S. Osuna, S. Filippone, M. Á. Herranz, Y. Murata, M. Solà and N. Martín, J. Am. Chem. Soc., 2015, 137, 1190–1197 CrossRef CAS PubMed; (b) E. E. Maroto, M. Izquierdo, M. Murata, S. Filippone, K. Komatsu, Y. Murata and N. Martín, Chem. Commun., 2014, 50, 740–742 RSC; (c) K. Sawai, Y. Takano, M. Izquierdo, S. Filippone, N. Martín, Z. Slanina, N. Mizorogi, M. Waelchli, T. Tsuchiya, T. Akasaka and S. Nagase, J. Am. Chem. Soc., 2011, 133, 17746–17752 CrossRef CAS PubMed.
- (a) J. Marco-Martínez, S. Reboredo, M. Izquierdo, V. Marcos, J. L. López, S. Filippone and N. Martín, J. Am. Chem. Soc., 2014, 136, 2897–2904 CrossRef PubMed; (b) J. Marco-Martínez, V. Marcos, S. Reboredo, S. Filippone and N. Martín, Angew. Chem., Int. Ed., 2013, 52, 5115–5119 CrossRef PubMed.
- (a) S. H. Laurie, in Comprehensive Coordination Chemistry, ed. G. Wilkinson, Pergamon, Oxford, 1987, vol. 2, ch. 20.2, p. 739 Search PubMed; (b) K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Wiley, New York, 4th edn, 1986, p. 233 Search PubMed.
- (a) W. Bauer, M. Prem, K. Polborn, K. Sünkel, W. Steglich and W. Beck, Eur. J. Inorg. Chem., 1998, 1998, 485–493 CrossRef; (b) D. Carmona, M. Pilar Lamata, F. Viguri, E. San José, A. Mendoza, F. J. Lahoz, P. García-Orduña, R. Atencio and L. A. Oro, J. Organomet. Chem., 2012, 717, 152–163 CrossRef CAS.
- See the ESI†.
- Fulleranes the Hydrogenated Fullerenes, ed., F. Cataldo and S. Iglesias-Groth, Springer Science+Business Media B.V., Dordrecht, 2010 Search PubMed.
- J. Nossal, R. K. Saini, L. B. Alemany, M. Meier and W. E. Billups, Eur. J. Org. Chem., 2001, 4167–4180 CrossRef CAS.
- G. W. Wang, Y. J. Li, F. B. Li and Y. C. Liu, Lett. Org. Chem., 2005, 2, 595–598 CrossRef CAS.
- R. M. Girón, S. Reboredo, J. Marco-Martínez, S. Filippone and N. Martín, Faraday Discuss., 2014, 173, 311–322 Search PubMed.
- C. Zhang and X. Lu, J. Org. Chem., 1995, 60, 2906–2908 CrossRef CAS.
- (a) A. Sánchez-Díaz, M. Izquierdo, S. Filippone, N. Martin and E. Palomares, Adv. Funct. Mater., 2010, 20, 2695–2700 CrossRef; (b) G. Garcia-Belmonte, P. P. Boix, J. Bisquert, M. Lenes, H. J. Bolink, A. La Rosa, S. Filippone and N. Martín, J. Phys. Chem. Lett., 2010, 1, 2566–2571 CrossRef CAS; (c) Y.-J. Cheng, M.-H. Liao, C.-Y. Chang, W.-S. Kao, C.-E. Wu and C.-S. Hsu, Chem. Mater., 2011, 23, 4056–4062 CrossRef CAS.
- (a) D. Cao, A. Wieckowski, J. Inukai and N. Alonso-Vante, J. Electrochem. Soc., 2006, 153, A869–A874 CrossRef CAS; (b) Y. Nie, L. Li and Z. Wei, Chem. Soc. Rev., 2015, 44, 2168–2201 RSC; (c) J. Qiao, R. Lin, B. Li, J. Ma and J. Liu, Electrochim. Acta, 2010, 55, 8490–8497 CrossRef CAS.
- (a) J. Qiao, M. Saito, K. Hayamizu and T. Okada, J. Electrochem. Soc., 2006, 15, A967–A974 CrossRef; (b) V. A. Sethuraman, J. W. Weidner, A. T. Haug, M. Pemberton and L. V. Protsailo, Electrochim. Acta, 2009, 54, 5571–5582 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details of the preparation of the fullerene derivatives, polymer blend absorption spectra, spectroscopic and chromatographic data for the characterization of compounds, linear scan voltammetry and Cyclic Voltammetry (CV) measurements. CCDC 960717. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ta06573b |
| This journal is © The Royal Society of Chemistry 2016 |