A nanoporous PtCuTi alloy with a low Pt content and greatly enhanced electrocatalytic performance towards methanol oxidation and oxygen reduction†
Abstract
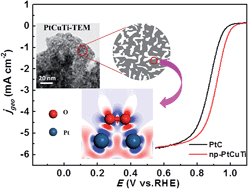
Pt-based electrocatalysts play a crucial role in both the anode and cathode reactions of direct methanol fuel cells (DMFCs), but their activity/durability and cost are still the main issues to be addressed. Through the combination of mechanical alloying with dealloying, here we have fabricated a nanoporous PtCuTi (np-PtCuTi) alloy with a low Pt content from a Cu-based precursor. The np-PtCuTi alloy exhibits a three-dimensional bi-continuous interpenetrating ligament/channel structure with a ligament size of 3.1 ± 0.6 nm. Electrochemical measurements show that the np-PtCuTi alloy exhibits superior electrocatalytic activities (CO tolerance, specific and mass activity) towards methanol oxidation at the anode, compared to commercial PtC catalysts. Moreover, the np-PtCuTi catalyst shows an enhancement of 1.9 and 4.2 times in the mass and specific activity towards the oxygen reduction reaction (ORR) at the cathode compared to PtC, respectively. More importantly, the np-PtCuTi catalyst shows excellent catalytic durability for the ORR, and the mass activity retains 91.8% of the initial value after 20 000 cycles. In addition, the mechanisms for the activity enhancement of np-PtCuTi have been rationalized on the basis of the structural effect, alloying effect and electronic effect through experiments and density functional theory calculations.

 Please wait while we load your content...
Please wait while we load your content...