Open Access Article
Open Access ArticleIn situ large-scale construction of sulfur-functionalized metal–organic framework and its efficient removal of Hg(II) from water†
Linfeng
Liang
abd,
Qihui
Chen
*ac,
Feilong
Jiang
ac,
Daqiang
Yuan
 ac,
Jinjie
Qian
ac,
Jinjie
Qian
 a,
Guangxun
Lv
a,
Hui
Xue
ac,
Luyao
Liu
a,
Hai-Long
Jiang
bd and
Maochun
Hong
*acd
a,
Guangxun
Lv
a,
Hui
Xue
ac,
Luyao
Liu
a,
Hai-Long
Jiang
bd and
Maochun
Hong
*acd
aState Key Lab of Structure Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, 350002, Fujian, China. E-mail: chenqh@fjirsm.ac.cn; hmc@fjirsm.ac.cn
bDepartment of Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, Hefei, Anhui 230026, P. R. China
cUniversity of the Chinese Academy of Sciences, Beijing, 100049, China
dDepartment of Chemistry, University of Science and Technology of China, Hefei, Anhui 230026, P. R. China
First published on 12th September 2016
Abstract
A new strategy that uses sulfur-functionalized metal organic frameworks (MOFs) for removal of Hg(II) from water has been developed. This strategy is based around a novel sulfur-functionalized MOF FJI-H12, which is composed of octahedral M6L4 cages and free NCS− groups. At the same time, large-scale synthesis of FJI-H12 microcrystals has also been carried out under very mild conditions. The resulting material can remove Hg(II) completely and selectively from water with high saturation, adsorption (439.8 mg g−1) and distribution coefficient (1.85 × 106 mL g−1) relative to other MOFs. More interestingly, a continuous and fast removal of Hg(II) from water has also been carried out using a column loaded with FJI-H12 microcrystals.
Introduction
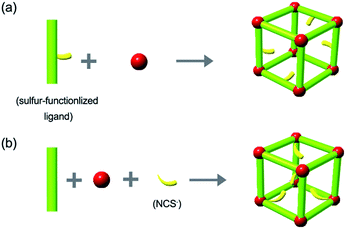
Mercury (Hg) pollution, which can cause birth defects, brain damage, and various other diseases in humans and animals, has become a serious threat to public health and the environment. Hence, the development of methods for its removal from waste water is highly urgent.1 A variety of adsorbents, such as activated carbons, zeolites and clays, have already been used for this purpose; however, such materials usually face challenges such as low surface area, low capacity, and moderate affinity for Hg(II).2 Metal–organic frameworks (MOFs), based on organic bridging ligands and metal ions or metal ion clusters, are a promising class of highly ordered, porous materials that have potential applications in gas storage, catalysis, and drug delivery, due to their unique properties such as permanent porosity and high crystallinity.3 Readily accessible external and internal surfaces and evenly distributed active sites through purposeful design make adsorption of heavy metal ions by MOFs possible.4 Methylthio groups were first introduced into MOFs to adsorb Hg(II) by Xu and coworkers.5 Many other functional groups have since been introduced to capture Hg(II) based on Hg–S interactions or Hg–N interactions. Sulfur-functionalized MOFs based on strong Hg–S interaction have proved particularly useful as sorbents for Hg(II).6The synthesis of sulfur-functionalized MOFs using preconstructed sulfur-functionalized ligands is illustrated in Scheme 1a. While this can be a powerful strategy for Hg(II) removal, it usually requires expensive reagents or harsh reaction conditions.7In situ introduction of sulfur-containing groups into MOFs based on coordination bonding might be an effective approach to overcome such challenges. The NCS− group is an ideal candidate for this route; it could be introduced into the framework during MOFs construction by coordination of the chemically hard N atom to a hard metal ion, while the chemically soft S atom remains available for the capture of heavy metal ions such as Hg(II) (Scheme 1b). Although NCS− has been used to construct discrete metallocages or MOFs,8 as far as we know, use of such material to capture Hg(II) has not been reported. Herein, a novel sulfur-functionalized MOF FJI-H12 was constructed using NCS−, Co(II) and 2,4,6-tri(1-imidazolyl)-1,3,5-triazine (Timt). The components were arranged in infinite octahedral M6L4 cages containing free NCS− groups. As we expected, N atoms of NCS− are coordinated to Co atoms, while the S atoms are free-standing. Moreover, large-scale synthesis of FJI-H12 microcrystals has also been realized under very mild conditions. Further researches demonstrate that such a material can remove Hg(II) from water completely and selectively. More interestingly, a continuous and fast removal of Hg(II) from water has been realized using a column loaded with FJI-H12 microcrystals.
Results and discussion
Syntheses and structures of FJI-H12
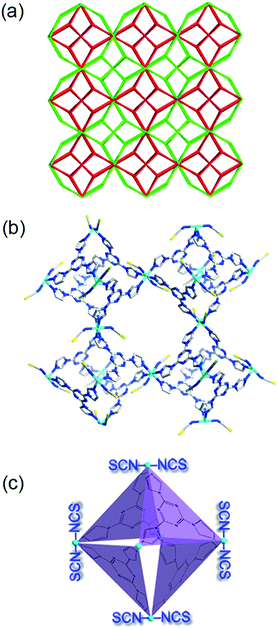
Layering an ethanol solution of Timt onto a water solution of K2Co(NCS)4 at room temperature for three days leads to the formation of light pink octahedral single crystals (denoted as FJI-H12), formulated as [Co3(Timt)4(SCN)6(H2O)14(EtOH)]n (for details, please refer to ESI S1†). Single crystal X-ray analysis reveals that FJI-H12 has a two-fold penetration structure and each fold comprises an infinite array of octahedral M6L4 cages, which are constructed by six Co(II) vertexes and four Timt panels (Fig. 1a and b). Apart from four imidazole rings, each Co(II) vertex of the M6L4 cage also bonds to two NCS− groups at their chemically hard N-sides, leaving their chemically soft S atoms pointing into the cavity (Fig. 1c).Metal ions selective adsorption test of FJI-H12
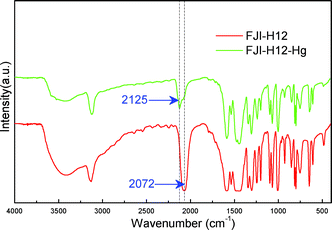
Although there is a two-fold penetration, FJI-H12 is still highly porous and this can be confirmed by single component low-pressure gas adsorption test. N2 adsorption isotherms at 77.4 K revealed its microporous nature (Fig. S1†). The pore size distribution ranges from 6.8 Å to 14.9 Å (Fig. S2†), which is far larger than the Hg(II) ion radius and obviously can guarantee the accessibility of free-standing S atoms for Hg(II) adsorption. Before metal adsorption testing, the chemical stability in various reagents and the thermal stability of FJI-H12 were investigated. We ultimately found that FJI-H12 is stable in water and various organic solvents, such as acetone, methanol, isopropanol, cyclohexane and ethanol (Fig. S3†), and it is thermally stable up to 200 °C (Fig. S4†).The metal selectivity tests were firstly performed on FJI-H12 in a 50 mL Hg(II) solution containing Mn(II), Ba(II), Ni(II), and Cd(II) background ions in high concentrations. Not only did FJI-H12 completely remove Hg(II) from the test sample, after which Hg(II) was not detected by ICP, but it also absorbed another highly toxic heavy metal ion, Cd(II). In contrast, other background metal ions, Mn(II), Ba(II) and Ni(II), were not adsorbed by FJI-H12 in a significant quantity (Table 1). The high selectivity of FJI-H12 for Hg(II) relative to the other metal ions such as Cd(II), Mn(II), Ba(II), and Ni(II) observed for FJI-H12 may result from the strong Hg–S interactions, which have been demonstrated by FT-IR studies. As shown in Fig. 2, the typical stretch mode of SCN− shows a large shift from 2072 cm−1 to 2125 cm−1 in FJI-H12–Hg (the FJI-H12 sample after Hg adsorption), indicating the strong binding interactions between Hg(II) and SCN−.
| a Treatment conditions: excess FJI-H12 (200 mg) was added to 50 mL water solution of metal ions and stirred at room temperature for 12 hours. The concentration of each metal is determined before and after treatment by Inductively Coupled Plasma (ICP). b The real value is less than the limit of detection of ICP instrument (0.02 ppb). | |||||
|---|---|---|---|---|---|
| Metal ion | Hg | Cd | Ni | Ba | Mn |
| Before treatment | 20.45 | 84.82 | 128.89 | 107.71 | 126.68 |
| After treatmenta | —b | 58.53 | 128.82 | 106.77 | 126.24 |
Hg(II) saturation adsorption amount of FJI-H12
To assess the mercury adsorption capacity of FJI-H12, which is also an important aspect of a sorbent's performance, an activated sample of FJI-H12 (100 mg) and 50 mL aqueous HgCl2 solution (397 mg HgCl2, 4 equivalents) were stirred together at room temperature for 12 h. The solid was then isolated by centrifugation and further washed with ethanol/H2O to remove residual HgCl2 on the exterior of the FJI-H12 powder sample. The solid sample thus obtained was subjected to regular inductively coupled plasma (ICP) analysis. Testing determined the Hg/Co ratio to be 1.20/1, corresponding to a maximum mercury adsorption capacity qmax of 439.8 mg g−1 (2.19 mmol g−1), a value which is very high relative to other MOF materials (Table 2). Further researches over the pH range of 3.0 to 6.0 demonstrate that FJI-H12 shows its highest adsorption capacity at pH = 7; when the pH is increased from 3.0 to 6.0, the removal efficiency capacity is basically unchanged (Fig. S5†).| MOF | q max, mg g−1 | K d, mL g−1 | Ref. |
|---|---|---|---|
| a The adsorption of Hg(II) from benzene. b The adsorption of Hg(II) from DMF. c The adsorption of Hg(II) from ethanol and the rest were processed in water. | |||
| Pb-TMBD | 361.3a | — | 5 |
| Zr-DMBD | 198.2 | 9.99 × 105 | 6e |
| Zn-hip | 278 | 1.11 × 106 | 6d |
| PCN-100 | 364.7b | — | 6a |
| PCN-101 | 103.9b | — | 6a |
| Zn4O(L)3 | 102.8c | 3.16 × 103 | 6b |
| FJI-H12 | 439.8 | 1.86 × 106 | This work |
Hg(II) adsorption kinetics
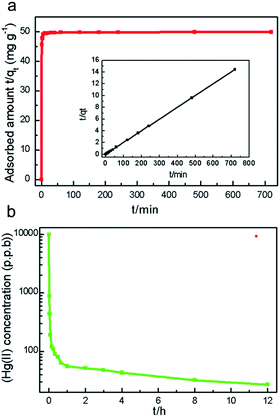
Given the high Hg(II) saturation level of this material, another test that focuses on its kinetic character has been carried out as follows: an activated FJI-H12 sample (10 mg) was placed in a dilute aqueous solution (50 mL) of HgCl2 (10 ppm). The residual Hg(II) concentration in the solution was determined at different intervals. Fig. 3a shows the fitting results of the experimental data for mercury adsorption on FJI-H12 by the pseudo-second-order kinetic model using the following equation:where qt (mg g−1) and qe (mg g−1) are the Hg(II) adsorption amounts at time t (min) and at equilibrium, respectively, and k2 (g mg−1 min−1) is the rate constant of pseudo-second order adsorption. An extremely high correlation coefficient (>0.9999) was obtained (Fig. 3b and the inset), suggesting that the pseudo-second-order model is suitable for describing the adsorption kinetics of this system. Furthermore, the calculated qe value from the pseudo-second-order model is in good agreement with the experimental value, qe,exp (Table S2†). Consequently, it is believed that mechanisms of both physisorption and chemisorption are involved in the current Hg(II) adsorption process, although to different degrees. Specifically, the SCN− groups in the pores hold the potential to coordinate with Hg(II) ions, and act as strong sites for Hg(II) chemisorptions.
The distribution coefficient (Kd) is always used to characterize a sorbent's affinity for a target metal ion, which is defined as follows:
Regeneration of FJI-H12
Whether an adsorbent can be regenerated is also an important aspect of its usefulness. To this point, a KSCN solution was used to regenerate a FJI-H12–Hg sample. By immersing FJI-H12–Hg in the KSCN solution (10 equivalents) at ambient temperature for 1 day, over 86% of the Hg(II) ions could be removed from FJI-H12–Hg powder (ICP analysis determined the Hg/Co ratio drops from 1.20/1 to 0.17/1 during this process), while the framework of FJI-H12 remains stable (Fig. S6†). Reuse of the regenerated FJI-H12 sample under the same conditions resulted in the corresponding Hg/Co ratio being reduced to 0.86/1, indicating that the efficiency of FJI-H12 is highly reduced after regeneration.Large-scale synthesis of FJI-H12 and continuous removal of Hg
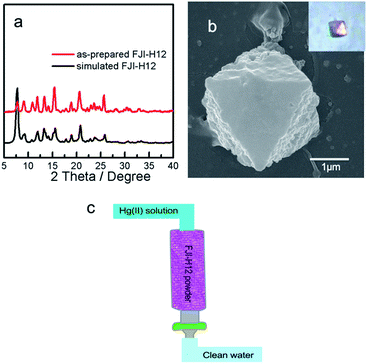
Several excellent sorbents based on strong Hg–S interactions have been developed; however, large-scale preparation and application of such materials are still highly challenging.6,10 After many attempts, a protocol for large-scale preparation of FJI-H12 has been established as follows: a high concentration water solution (1 mol L−1, 0.3 mL) of K2Co(SCN)4 was added to a vigorously stirred ethanol solution (0.02 mol L−1, 20 mL) of Timt, immediately leading to the formation of a uniform microcrystalline powder of FJI-H12. Its purity has been confirmed by powder X-ray diffraction (PXRD) analysis (Fig. 4a). The median microcrystal grain size was determined by laser diffraction analysis to be X50 = 2.1 μm (Fig. S7†). Scanning electron microscopy measurements also confirmed the formation of relatively uniform octahedral crystallites on the scale of about 2 μm (Fig. S8†). Moreover, the observed crystalline morphology is in accordance with the large single crystal obtained by the solution diffusion method (Fig. 4b and the inset). Further researches indicated that up to 10 g FJI-H12 could be prepared by this method (Fig. S6†).Since large-scale synthesis of FJI-H12 has been realized under very mild conditions, this makes possible the removal of Hg(II) from water on a large scale. A column for removal of Hg(II) from water was prepared by loading 2 g of FJI-H12 microcrystal powder into a 10 mL syringe equipped with a 0.22 μm Millipore filter (schematic in Fig. 4c). A 20 ppm Hg solution in 50 mL water could be completely purified by this column at a flow rate of 2 mL min−1. This represents the first continuous and fast removal of Hg(II) from water based on sulfur-functionalized MOF. It also implies the practicality of such materials for the waste-water treatment in industry.
Conclusions
In conclusion, a novel sulfur-functionalized metal–organic framework FJI-H12 has been synthesized for the capture of Hg(II) from water. Moreover, its microcrystalline powder can be synthesized up to 10 g scale within several minutes under very mild condition. Such material can remove Hg(II) completely and selectively from water with high saturation (439.8 mg g−1) and distribution coefficient (1.85 × 106 mL g−1) as compared to other MOF materials. More interestingly, a continuous and fast removal of Hg(II) from water has also been carried out using a column loaded with FJI-H12 microcrystalline powder. Thus, in situ construction of sulfur-functionalized MOF based on the SCN− group provides a new perspective for decontaminating Hg(II) from aqueous media and makes both large-scale synthesis and application of sulfur-functionalized MOF for Hg(II) capture more practical.Acknowledgements
We are thankful for financial support from the 973 Program (2014CB932101, 2013CB933200), the “Strategic Priority Research Program” of the Chinese Academy of Sciences (XDA09030102, XDA07070200), and the National Natural Science Foundation of China (21471148, 21131006).Notes and references
- (a) N. Lubick and D. Malakoff, Science, 2013, 341, 1443–1445 CrossRef CAS PubMed; (b) M. McNutt, Science, 2013, 341, 1430 CrossRef CAS PubMed; (c) C. Wang, S. Tao, W. Wei, C. Meng, F. Liu and M. Han, J. Mater. Chem., 2010, 20, 4635–4641 RSC.
- (a) U. Wingenfelder, C. Hansen, G. Furrer and R. Schulin, Environ. Sci. Technol., 2005, 39, 4606–4613 CrossRef CAS PubMed; (b) A. Benhammou, A. Yaacoubi, L. Nibou and B. Tanouti, J. Colloid Interface Sci., 2005, 282, 320–326 CrossRef CAS PubMed; (c) G. Blanchard, M. Maunaye and G. Martin, Water Res., 1984, 18, 1501–1507 CrossRef CAS; (d) C. P. Huang and D. W. Blankenship, Water Res., 1984, 18, 37–46 CrossRef CAS.
- (a) B. Li, M. Chrzanowski, Y. Zhang and S. Ma, Coord. Chem. Rev., 2016, 307, 106–129 CrossRef CAS , part 2; (b) L. Ma, C. Abney and W. Lin, Chem. Soc. Rev., 2009, 38, 1248–1256 RSC; (c) J.-P. Zhang, Y.-B. Zhang, J.-B. Lin and X.-M. Chen, Chem. Rev., 2012, 112, 1001–1033 CrossRef CAS PubMed; (d) Y. Cui, Y. Yue, G. Qian and B. Chen, Chem. Rev., 2012, 112, 1126–1162 CrossRef CAS PubMed; (e) D. Zhao, D. J. Timmons, D. Yuan and H.-C. Zhou, Acc. Chem. Res., 2011, 44, 123–133 CrossRef CAS PubMed; (f) P. Silva, S. M. F. Vilela, J. P. C. Tome and F. A. Almeida Paz, Chem. Soc. Rev., 2015, 44, 6774–6803 RSC; (g) Q. Yang, D. Liu, C. Zhong and J.-R. Li, Chem. Rev., 2013, 113, 8261–8323 CrossRef CAS PubMed; (h) S. M. Cohen, Chem. Rev., 2012, 112, 970–1000 CrossRef CAS PubMed; (i) J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC; (j) P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2012, 112, 1232–1268 CrossRef CAS PubMed; (k) R. C. Huxford, J. Della Rocca and W. Lin, Curr. Opin. Chem. Biol., 2010, 14, 262–268 CrossRef CAS PubMed; (l) M. Eddaoudi, D. F. Sava, J. F. Eubank, K. Adil and V. Guillerm, Chem. Soc. Rev., 2015, 44, 228–249 RSC; (m) J. J. I. V. Perry, J. A. Perman and M. J. Zaworotko, Chem. Soc. Rev., 2009, 38, 1400–1417 RSC; (n) M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2012, 112, 675–702 CrossRef PubMed; (o) M. P. Suh, H. J. Park, T. K. Prasad and D.-W. Lim, Chem. Rev., 2012, 112, 782–835 CrossRef CAS PubMed; (p) H. Wu, Q. Gong, D. H. Olson and J. Li, Chem. Rev., 2012, 112, 836–868 CrossRef CAS PubMed; (q) T. Uemura, N. Yanai and S. Kitagawa, Chem. Soc. Rev., 2009, 38, 1228–1236 RSC; (r) K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724–781 CrossRef CAS PubMed; (s) A. Dhakshinamoorthy, A. M. Asiri and H. Garcia, Chem. Soc. Rev., 2015, 44, 1922–1947 RSC; (t) Y. Yan, S. Yang, A. J. Blake and M. Schroeder, Acc. Chem. Res., 2014, 47, 296–307 CrossRef CAS PubMed; (u) K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724–781 CrossRef CAS PubMed; (v) S. S. Han, J. L. Mendoza-Cortes and W. A. Goddard Iii, Chem. Soc. Rev., 2009, 38, 1460–1476 RSC.
- (a) M. Carboni, C. W. Abney, S. Liu and W. Lin, Chem. Sci., 2013, 4, 2396–2402 RSC; (b) J. Cui, Y. L. Wong, M. Zeller, A. D. Hunter and Z. Xu, Angew. Chem., Int. Ed., 2014, 53, 14438–14442 CrossRef CAS PubMed; (c) J. He, M. Zha, J. Cui, M. Zeller, A. D. Hunter, S.-M. Yiu, S.-T. Lee and Z. Xu, J. Am. Chem. Soc., 2013, 135, 7807–7810 CrossRef CAS PubMed; (d) M. Zha, J. Liu, Y.-L. Wong and Z. Xu, J. Mater. Chem. A, 2015, 3, 3928–3934 RSC; (e) Y. Wang, G. Ye, H. Chen, X. Hu, Z. Niu and S. Ma, J. Mater. Chem. A, 2015, 3, 15292–15298 RSC; (f) Y. Wang, Z. Liu, Y. Li, Z. Bai, W. Liu, Y. Wang, X. Xu, C. Xiao, D. Sheng, J. Diwu, J. Su, Z. Chai, T. E. Albrecht-Schmitt and S. Wang, J. Am. Chem. Soc., 2015, 137, 6144–6147 CrossRef CAS PubMed; (g) X. Meng, R.-L. Zhong, X.-Z. Song, S.-Y. Song, Z.-M. Hao, M. Zhu, S.-N. Zhao and H.-J. Zhang, Chem. Commun., 2014, 50, 6406–6408 RSC; (h) H. Xue, Q. Chen, F. Jiang, D. Yuan, G. Lv, L. Liang, L. Liu and M. Hong, Chem. Sci., 2016, 7, 5983–5988 RSC.
- X. P. Zhou, Z. Xu, M. Zeller and A. D. Hunter, Chem. Commun., 2009, 5439–5441 RSC.
- (a) Q. R. Fang, D. Q. Yuan, J. Sculley, J. R. Li, Z. B. Han and H. C. Zhou, Inorg. Chem., 2010, 49, 11637–11642 CrossRef CAS PubMed; (b) J. He, K.-K. Yee, Z. Xu, M. Zeller, A. D. Hunter, S. S.-Y. Chui and C.-M. Che, Chem. Mater., 2011, 23, 2940–2947 CrossRef CAS; (c) T. Liu, J. X. Che, Y. Z. Hu, X. W. Dong, X. Y. Liu and C. M. Che, Chem.–Eur. J., 2014, 20, 14090–14095 CrossRef CAS PubMed; (d) F. Luo, J. L. Chen, L. L. Dang, W. N. Zhou, H. L. Lin, J. Q. Li, S. J. Liu and M. B. Luo, J. Mater. Chem. A, 2015, 3, 9616–9620 RSC; (e) K. K. Yee, N. Reimer, J. Liu, S. Y. Cheng, S. M. Yiu, J. Weber, N. Stock and Z. Xu, J. Am. Chem. Soc., 2013, 135, 7795–7798 CrossRef CAS PubMed.
- (a) S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400–5449 CrossRef CAS PubMed; (b) T. Kondo and T.-A. Mitsudo, Chem. Rev., 2000, 100, 3205–3220 CrossRef CAS PubMed; (c) L. Vial, R. F. Ludlow, J. Leclaire, R. Pérez-Fernández and S. Otto, J. Am. Chem. Soc., 2006, 128, 10253–10257 CrossRef CAS PubMed.
- (a) M. B. Duriska, S. M. Neville, J. Lu, S. S. Iremonger, J. F. Boas, C. J. Kepert and S. R. Batten, Angew. Chem., Int. Ed., 2009, 48, 8919–8922 CrossRef CAS PubMed; (b) S. M. Neville, B. Moubaraki, K. S. Murray and C. J. Kepert, Angew. Chem., Int. Ed., 2007, 46, 2059–2062 CrossRef CAS PubMed; (c) Y. Inokuma, T. Arai and M. Fujita, Nat. Chem., 2010, 2, 780–783 CrossRef CAS PubMed.
- Y. Shin, G. E. Fryxell, W. Um, K. Parker, S. V. Mattigod and R. Skaggs, Adv. Funct. Mater., 2007, 17, 2897–2901 CrossRef CAS.
- (a) S.-Y. Ding, M. Dong, Y.-W. Wang, Y.-T. Chen, H.-Z. Wang, C.-Y. Su and W. Wang, J. Am. Chem. Soc., 2016, 138, 3031–3037 CrossRef CAS PubMed; (b) B. Li, Y. Zhang, D. Ma, Z. Shi and S. Ma, Nat. Commun., 2014, 5, 5537 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1474064. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ta04927c |
| This journal is © The Royal Society of Chemistry 2016 |