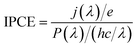Open Access Article
Open Access ArticleBulky crystalline BiVO4 thin films for efficient solar water splitting
Miao
Zhong
ab,
Takashi
Hisatomi
ab,
Tsutomu
Minegishi
 ab,
Hiroshi
Nishiyama
ab,
Masao
Katayama
ab,
Taro
Yamada
ab and
Kazunari
Domen
*ab
ab,
Hiroshi
Nishiyama
ab,
Masao
Katayama
ab,
Taro
Yamada
ab and
Kazunari
Domen
*ab
aDepartment of Chemical Systems Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan. E-mail: domen@chemsys.t.u-tokyo.ac.jp
bJapan Technological Research Association of Artificial Photosynthetic Chemical Process, 2-11-9 Iwamotocho, Chiyoda-ku, Tokyo 101-0032, Japan
First published on 25th May 2016
Abstract
We report a new fabrication method of flat, uniform BiVO4 films on an electrically conductive transparent indium tin oxide (ITO) film based on a solution process for depositing bismuth precursor films and a high-temperature calcination process with an organic vanadium precursor. The synthesised BiVO4 films, composed as a monolayer of crystallites (diameter ≤ 1 μm) fixed on the ITO, realised half-cell solar-to-hydrogen energy conversion efficiencies of over 1.5% by the aid of impregnated CoOx and atomic-layer-deposited NiO when tested as oxygen-evolving photoanodes for water splitting under solar simulator AM 1.5G illumination. Stoichiometric oxygen and hydrogen were generated with Faraday efficiencies of unity over 12 h at 0.6–0.9 VRHE. This morphology of our bulky semi-transparent BiVO4 films exhibited state-of-the-art solar water splitting performances.
Introduction
Photoelectrochemical (PEC) water splitting — harvesting solar energy to produce clean and renewable hydrogen fuels from water — is a promising technique to fulfil the increasing energy demands of modern society with minimum environmental impacts.1–3 Considerable efforts have been made to realize high-performance and low-cost PEC overall water splitting devices. In PEC hydrogen evolution reactions (HERs), high solar energy conversion efficiencies are achieved when p-type semiconductors that are excellent light absorbers with high carrier mobility are used in photocathode-based half-cell PEC devices.2,4 However, the development of efficient and stable photoanodes for oxygen evolution reactions (OERs) is still a challenging field.One essential problem that impedes photoanode efficiency is the high activation energy associated with the complex four-electron OER process.5 Holes easily recombine at photoanode/electrolyte interfaces before they oxidise water. Earth-abundant CoPi6 and Ni/Fe layered-double-hydroxide (LDH)7,8 have been developed as robust catalysts to largely improve OER kinetics in bias-driven PEC electrolysis. Besides the decoration of effective OER catalysts, suppression of charge recombination at photoanode surfaces by conformal coating of p-type materials has been reported.8,9 In this way, dangling bonds at photoanode surfaces are passivated and buried p/n junctions are created to increase the hole lifetime.
Suppression of charge recombination in the photoanode bulk is another fundamental aspect for improving PEC performances. Photoanode materials that can effectively separate charges and efficiently transport holes are required. BiVO4 is one of the most attractive candidates,10–12 due to its deep valence band for OER under visible light and long-term stability in near-neutral aqueous solutions. Further, BiVO4 has a long carrier lifetime of ∼40 ns compared to that of <10 ps for other oxide photocatalysts such as hemitate.13,14 However, the carrier mobility in BiVO4 is low at ∼0.044 cm2 V−1 s−1, indicating that most of the photo-generated holes are localised in BiVO4.
To achieve efficient charge separation in BiVO4, attempts have been made on fabricating (1) monolayer BiVO4 sub-micro nanoparticles on conducting Ti metal by particle transfer methods,8 (2) BiVO4 homo-/hetero-junctions such as BiVO4/WO3/ITO films10,14 and (3) nanostructures such as nanowire arrays.15,16 Recently, Choi et al. reported nanoporous BiVO4 with state-of-the-art photocurrent density and efficiency.12 A simple and effective electrodeposition–annealing process was applied for synthesizing nanoporous BiVO4 with particle sizes of ∼80 nm. Nanostructures drastically improve charge separation and surface areas for largely enhanced PEC performances.
However, nanostructures have disadvantages that warrant special care with respect to their practical applications. (1) Their mechanical strength is not satisfactory and achieving mechanical robustness is a stubborn challenge for practical operation. It is difficult to improve the mechanical strength of certain materials, especially nanostructured materials. (2) Coating of other functional materials over entire nanostructures is also difficult compared to that for flat thin films. This further hampers the construction of tandem devices when starting with nanostructures.
In contrast, multi-layered tandem film devices with anti-reflective coatings are commercialised for solar cells. Thus also for PEC applications aiming for future industrialisation, it is desirable to fabricate robust and flat BiVO4 thin films with exact stoichiometry, the most photoactive crystallographic structure, and high crystallinity with the particle size optimised.
In this study, we present an anodic electrodeposition and calcination process for fabricating bulky BiVO4 crystalline films on transparent ITO glass for use as photoanodes in solar water splitting cells. The fabricated films were proven to be mechanically robust. After surface decoration with CoOx catalysts and conformal deposition of NiO layers, a half-cell solar-to-hydrogen (HC-STH) conversion efficiency of over 1.5% was realised. Stoichiometric hydrogen and oxygen were generated with unity Faraday efficiency over 12 h.
Experimental
Deposition of Bi precursor thin films
First, 25 mL 0.1 M Bi(NO3)3 in acetic acid was prepared. The pH of the prepared solution was adjusted to 4.8 by adding 5 M NaOH aqueous solution. Then 20 mL 0.3 M p-benzoquinone in ethanol (99.95%) was added into the solution. After gently stirring for 1 h, the colour of the solution changed to transparent dark brown. Electrodeposition in this mixture was performed on ITO glass plates (Geomatec, sheet resistivity ∼ 5 Ω sq−1, 30 mm × 10–30 mm × 1 mm thickness). A potentiostat (Hokuto Denko, HSV-100) was used at room temperature with ITO glass as the working electrode, an Ag/AgCl reference electrode and a Pt counter electrode. The ITO working electrode was maintained at 2.3 VAg/AgCl. The optimised deposition time was 7 min.Synthesis of BiVO4 thin films
The Bi precursor film was drop-painted with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dimethyl sulfoxide/ethanol solution of vanadyl diacetylacetonate (VO(acac)2, 0.2 M). The casting amount was 0.075 mL for a 20 mm × 20 mm Bi precursor film. The VO(acac)2/Bi precursor film was calcined in a muffle furnace in air at various temperatures from 490–530 °C. The excess amount of VOx was washed away in 1 M NaOH solution for 10 min with gentle stirring. The obtained BiVO4 films were then cleaned with DI water and dried at room temperature. In our experiments, BiVO4 films synthesised at 520 °C for 2 h with the initial temperature ramping rate of 2 °C min−1 exhibited the best performance.
1 dimethyl sulfoxide/ethanol solution of vanadyl diacetylacetonate (VO(acac)2, 0.2 M). The casting amount was 0.075 mL for a 20 mm × 20 mm Bi precursor film. The VO(acac)2/Bi precursor film was calcined in a muffle furnace in air at various temperatures from 490–530 °C. The excess amount of VOx was washed away in 1 M NaOH solution for 10 min with gentle stirring. The obtained BiVO4 films were then cleaned with DI water and dried at room temperature. In our experiments, BiVO4 films synthesised at 520 °C for 2 h with the initial temperature ramping rate of 2 °C min−1 exhibited the best performance.
Loading of CoOx on BiVO4
The BiVO4 films were immersed in a 20 mL 0.01 M Co(NO3)2 and 0.01 M NH3 H2O solution at pH 8.4 for 0.5 h. Then the films were washed with DI water and annealed in air at 250 °C for 0.5 h.Atomic-layer deposition of NiO on CoOx/BiVO4
Atomic-layer deposition (ALD) was applied to deposit NiO on the CoOx/BiVO4 films. Bis-(2,2,6,6-tetramethylheptane-3,5-dionato) nickel(II) (Ni(thd)2 in short) and H2O were used as the precursors. Ni(thd)2 was heated to 165 °C and H2O was set to 15 °C. The temperature in the deposition chamber was 260 °C. One ALD cycle consists of an H2O pulse for 6 s, N2 purging for 3 s, pressurising the Ni(thd)2 container by N2 gas (500 sccm) for 3 s, a Ni(thd)2/N2 pulse for 6 s, and a N2 purging for 3 s. Before ALD, CoOx/BiVO4 was treated in an ozone plasma cleaning machine for 5 min to increase surface wettability with respect to hydroxyl ions. For BiVO4 films, 300 cycles ALD delivered the best performances. Excess deposition of NiO on CoOx/BiVO4 could be thinned by 50 mV s−1 cyclic-voltammetry scans from −0.6 to 0.8 VAg/AgCl in a 1 M pH 6 Na2SO4 solution.Characterizations
Scanning electron microscopic (SEM) observations were carried out using a Hitachi SU8020 system. The X-ray diffraction (XRD) was measured by a SmartLab XRD (Rigaku, Japan). The X-ray wavelength was 0.15418 nm (Cu Kα).The PEC performances were investigated using a three-electrode electrochemical configuration in a 0.5 M potassium borate (K3BO3) buffer solution at pH = 9.5 by a solar simulator light source (SAN-EI electronic, XES40S1). The light intensity of this solar simulator was adjusted to AM 1.5G (100 mW cm−2) by measuring with a calibrated photometer (Hamamatsu model S2281). The electrolyte was stirred and bubbled with Ar gas before and during measurements. An Ag/AgCl electrode in saturated KCl solution was used as a reference electrode and a Pt coil was used as the counter electrode. The potential unit is converted to the reversible hydrogen electrode according to the Nernst equation,
| VRHE = VAg/AgCl + 0.059pH + V0Ag/AgCl |
| V0Ag/AgCl = 0.199 V at 25 °C |
The incident light spectral analysis for the photocurrent was performed using a Xe lamp (Asahi Spectra, MAX-302) equipped with bandpass filters (central wavelengths: 400–540 nm, every 20 nm; full width at half maximum: 10 nm). The irradiance spectra of the light incident on the electrode surface were measured with a spectroradiometer (EKO Instruments, LS-100, absolutely calibrated for the intensity).
The gas chromatographic (GC) quantification of H2 and O2 during the PEC H2O splitting was performed with a dedicated setup. An air-tight three-electrode PEC cell with NiO/CoOx/BiVO4 working electrodes, Ag/AgCl reference electrodes and Cr-coated Pt counter electrodes was used for GC analyses. Here, Cr was coated on Pt wire to suppress the reverse reaction of oxygen reduction on the Pt wire in solution. The PEC cell was connected to a vacuum pump and a micro GC (Inficon Co., Ltd, 3000). Before measurement, the PEC cell was pumped to a low vacuum and then purged with Ar sufficiently to get rid of N2 and O2 gases in the PEC cell. H2 and O2 evolution were measured in 0.5 M K3BO3 buffer solution at pH 9.5 under simulated sunlight illumination for 12 h with potential vs. RHE at 0.6 and 0.9 VRHE. The electric current passing through the outer circuit was recorded by a potentiostat (Princeton Research, VersaSTAT 3000) during H2 and O2 evolution reactions. The GC peak areas for H2 and O2 were converted to μmol using calibration curves, obtained by measuring the same amounts of H2 and O2 gases pre-injected in the PEC cell. The sampling of the gas in the PEC cell for GC was performed every 20 min.
Results and discussion
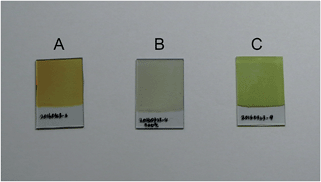
Fig. 1 shows photographs of ITO glass plates covered by an as-deposited Bi precursor film, a Bi precursor film annealed at 500 °C, and the BiVO4 film prepared according to the above-mentioned procedures. We temporarily call the precursor film BiCxOy. The as-deposited BiCxOy film might be hydrated and contain carbonate and other carbonaceous components. Bubbles were seen when this film was immersed in dilute HCl, and the gas was probably CO2. During the electrochemical deposition process, CH3COO− in pH 5–6 solution can be altered into CO32− by Kolbe electrolysis. The mechanism of Bi accumulation on this positively polarised electrode must include elementary reactions involving CH3COO−, which is the major component of this solution.Annealing the BiCxOy film in a muffle furnace in air altered the colour of the film. Before annealing, the as-deposited BiCxOy film looks brown (Fig. 1A), and after annealing at 500 °C the colour is close to white (Fig. 1B). The change in colour is related to the changes in the Bi compounds in the film. XRD spectra were recorded for the films from as-deposited BiCxOy and annealed BiCxOy, shown in Fig. 2B–E. The spectra always contain diffractions from the substrate ITO (A). The as-deposited BiCxOy and annealed samples below 300 °C exhibit broad scattering at 28° of the diffraction angle (Fig. 2B). The films were amorphous, probably composed of nano-crystallites of Bi carbonaceous compounds. The components of BiCxOy might be those such as bismuth carbonate, bismuth hydroxide carbonate18,19 and other carbonaceous materials. Above the annealing temperature of 300 °C, XRD exhibited sharp peaks (Fig. 2D and E), better matching the standard peak positions of crystalline BiO than those of Bi2O3 (Fig. 2 top). We carried out elemental analysis of the as-deposited BiCxOy film and BiCxOy film annealed at 500 °C, having scratched them off from the ITO glass. This powder was subjected to decomposition in HNO3 and Bi quantification by means of inductively coupled plasma (ICP) spectroscopy. The Bi contents for as-deposited BiCxOy film and BiCxOy film annealed at 500 °C were 76.8% w/w and 86.4% w/w, respectively.
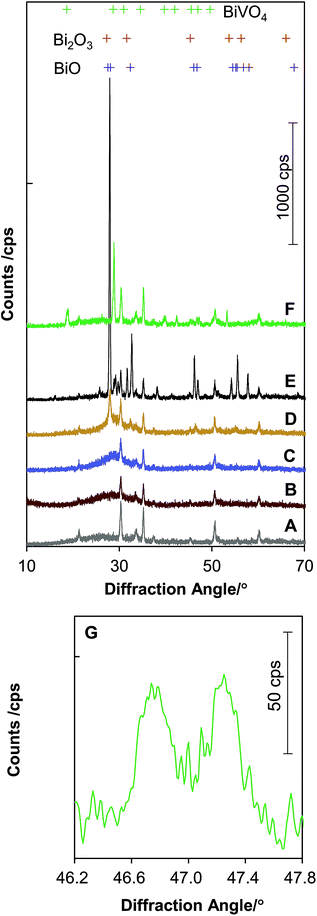 | ||
| Fig. 2 X-ray diffraction spectra of (A) a bare ITO glass piece (Geomatec, ITO thickness ∼ 500 nm, sheet resistivity ∼ 5 Ω sq−1), (B) the as-deposited BiCxOy, (C) BiCxOy heated at 180 °C in air, (D) at 300 °C, (E) at 500 °C, (F) BiVO4 film by calcination of as-deposited BiCxOy, and VO(acac)2 at 520 °C for 1 h, (G) an expanded spectrum from curve (F) for (024) and (204) diffractions of the scheelite monoclinic BiVO4. The X-ray wavelength was 0.15418 nm (Cu Kα). The standard diffraction peak positions are indicated by + at the top, referring to ref. 25 for BiO, ref. 26 for Bi2O3 and ref. 27 for BiVO4. | ||
The annealed film is compositionally close to BiO (Bi 92.9% w/w) or Bi2O3 (Bi 89.7% w/w). Although there were some residual carbonaceous materials therein, the films annealed at 500 °C were determined to be mainly composed of crystalline BiO.
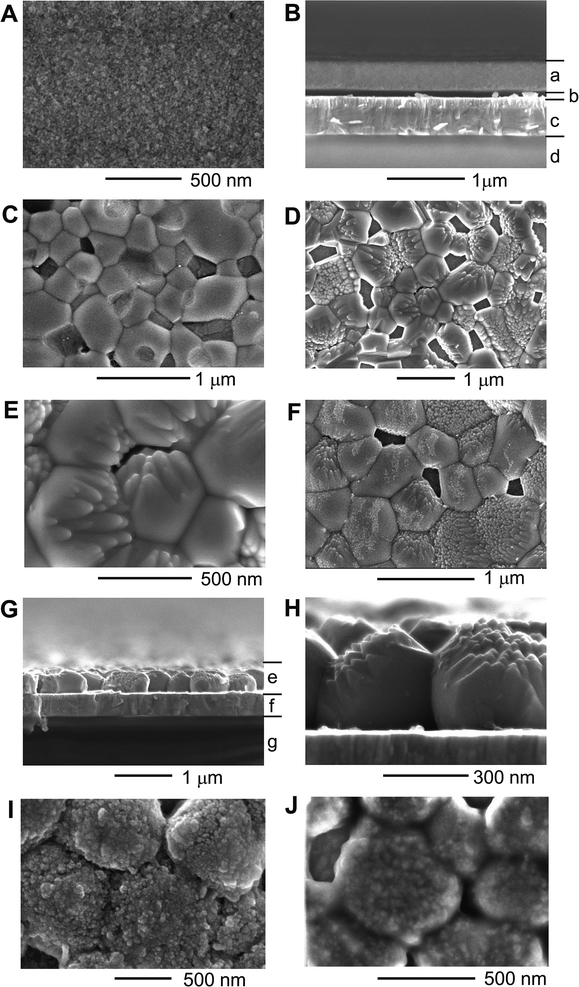
The morphological change of BiCxOy upon annealing is visualised by scanning electron microscopy, as shown in Fig. 3. The original as-deposited BiCxOy comprised a flat, smooth, homogeneous layer with a thickness of ∼300 nm (Fig. 3A and B). The top view indicates that the film consists of fine grains (diameter < 10 nm). As annealing temperature was raised and reached 500 °C, the grain size increased up to 1 μm (Fig. 3C). The grains compose a monolayer over the ITO surface and some voids are seen surrounded by grains. This bulky morphology of BiO particles is the prototype of BiVO4 films, as discussed later.
Calcination of the as-deposited BiCxOy with vanadyl bis-acetylacetonate (VO(acac)2) at 520 °C formed highly crystalline BiVO4 of scheelite monoclinic type, as seen in the XRD spectra in Fig. 2F and G. Splitting of the BiVO4 (024) and (204) peaks at 46.7° and 47.3° (Fig. 2B) was clearly observed. This is essentially different from the single (024) peak of ∼47° for tetragonal BiVO4.21 Note that there is no ITO peak between 46° and 48°, which rules out the other possibility for this XRD peak splitting. BiVO4 films calcined at different temperatures between 490 °C and 530 °C were all of the same monoclinic structure. The synthesised BiVO4 is made of large BiVO4 particles with an in-plane diameter of ∼1 μm and a thickness of ∼300 nm (Fig. 3D and E). Some grains, probably depending upon the crystallographic orientation, have a bunch of needle-like shapes aligned along one direction.
It is notable that the final BiVO4 particles (Fig. 3D and E) look quite similar in size and in shape to the BiO particles formed by annealing at 500 °C (Fig. 3C). Actually BiCxOy pre-annealed at 500 °C was subjected to calcination with VO(acac)2 at 520 °C. The same shape of BiVO4 crystallites (Fig. 3F) appeared as seen in Fig. 3D and E. Presumably the temperature ramping of BiCxOy + VO(acac)2 from room temperature induces the formation of BiO crystallites near 500 °C, and the BiO crystallites are converted into BiVO4 crystallites simultaneously with diffusion of V.17–20
The BiVO4 crystallites formed a particle monolayer firmly fixed on ITO. The in-plane diameter of BiVO4 crystallites is larger than that of the previously reported BiVO4 nanostructures or films synthesised by spray pyrolysis,11 electrochemical processes,12 chemical vapour deposition22 or co-evaporation.23
The two-step synthesis of BiVO4 was first reported by Choi and co-workers using BiOI as a starting material.12 The key step in their synthesis is the fabrication of very thin BiOI nanosheets that have a large surface area to make the Bi and V precursors be sufficiently in contact with each other for uniform BiVO4 nucleation during calcination. Differently from their BiOI cathodic deposition, we fabricated a dense Bi precursor film on ITO as anodes. The Bi precursor film is advantageous for the fabrication of bulky crystalline BiVO4 films, evidenced by the conversion from BiO crystallites observed by XRD and SEM analyses as described above.
The present BiVO4 films were mechanically robust. The film never came off even when subjected to pressing, rubbing or scratching by a finger or wiping with tissue paper. More systematically, we performed a peeling-off test with adhesive tape. A piece of adhesive tape (“mending tape”) was applied on the films, and pressed by loading a weight over the tape at 220 g cm−2 for 1 min. Then the tape was peeled off and the glue surface of the tape was inspected. In the case of present BiVO4 films, nothing looked transferred to the adhesive. The nanoporous films we reproduced according to the recipe12 did not survive these tests and released BiVO4 dust. This demonstrates the high reliability of this BiVO4 crystalline monolayer towards usage under mechanically severe conditions, such as in a flow of electrolytic solutions or in repetitive heating/cooling cycles.
As for the O2-evolution cocatalysts, CoOx was loaded on BiVO4 films by impregnation, and atomic layer deposition (ALD) was performed to deposit NiO on the surface of CoOx-loaded BiVO4. After PEC measurement, NiOOH was formed on the top surfaces of NiO nanoparticles (Fig. 3J). This state of the NiOOH are supposed to contain a large amount of surface hydroxyl ions for efficient OERs.7,8 Ultrathin NiOOH can allow penetration of hydroxyl ions and also enable hydroxyl ions to be in contact with the buried CoOx for a dual OER catalyst effect.7,8,12 A small amount of Co incorporation in NiOOH can also possibly increase its electric conductivity to reduce applied potential loss within the NiOOH layer, for realising high PEC performances.7,12,24
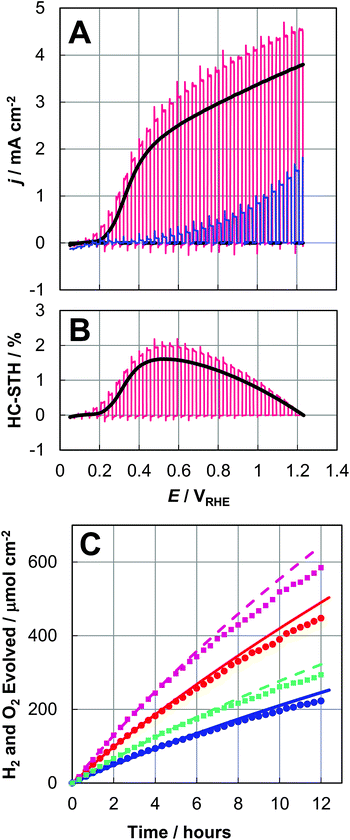
The PEC performance of the bare BiVO4 and NiO/CoOx/BiVO4 photoanodes was measured in 0.5 M pH 9.5 potassium borate (K3BO3) buffer solutions under simulated air mass (AM) 1.5G illumination. Fig. 4A shows the photocurrent density (j) as a function of the electrode potential (E). The chopped light blue and red j–E curves represent the PEC performances of our bare BiVO4 electrode and our best NiO/CoOx/BiVO4 electrode, respectively. The best electrode had a light acceptance/electrolytically active area of 0.95 cm2. The black j–E curve is an average PEC performance from j–E curves obtained with 10 different NiO/CoOx/BiVO4 test samples, with the average dark current also shown. We take this average data as the representative characteristics of the present NiO/CoOx/BiVO4 photoanode. The photocurrent onset potential was ∼0.2 VRHE in Fig. 4A. The anodic photocurrent at this low applied-bias region indicates the effective separation of photogenerated charges and efficient migration of holes towards BiVO4 surfaces for OERs. The photocurrent density is over 2.5 mA cm−2 at 0.6 VRHE for the NiO/CoOx/BiVO4 photoanode. The HC-STH curves, shown in Fig. 4B, are calculated from the j–E curves by
| HC-STH = (1.23E/VRHE)j/(solar simulator power/W cm−2) |
The average HC-STH curve reaches the maximum of 1.5% at 0.56 VRHE, which was markedly high among the reported single-photon photoanodes. This high performance of the semi-transparent BiVO4 films is suitable for the construction of parallel electrode devices, such as integrated solar water splitting devices with a narrow-bandgap photocathode or photovoltaic cells placed behind BiVO4/ITO/glass to utilize the transmitted solar photons through this semi-transparent electrode.
To confirm HERs at the Pt electrode and OERs at the NiO/CoOx/BiVO4 photoanode, the evolved gas was analysed by gas chromatography. The plots in Fig. 4C indicate the amounts of accumulated H2 and O2 gases within the PEC reactor measured by gas chromatography. The curves are obtained by integrating the photocurrent density as a function of time and converted into the accumulated amounts of H2 and O2 under the assumption that the photocurrent was completely consumed at the Pt cathode with 2 electrons for one H2 molecule and at the BiVO4 photoanode with 4 electrons for one O2 molecule. The NiO/CoOx/BiVO4 photoanodes were held at constant potentials of 0.6 and 0.9 VRHE in three-electrode configurations for 12 h. The evolution of H2 and O2 with a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was continuously demonstrated. The Faraday efficiency of HERs and OERs were both close to 100%, demonstrating that the photocurrent is all attributed to OERs and HERs.
1 was continuously demonstrated. The Faraday efficiency of HERs and OERs were both close to 100%, demonstrating that the photocurrent is all attributed to OERs and HERs.
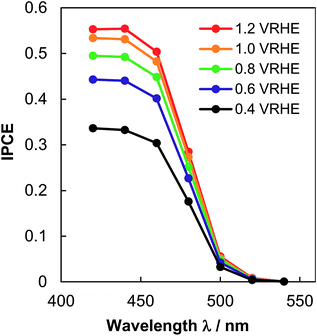
The efficiency of the NiO/CoOx/BiVO4 photoanode was verified by measuring the wavelength dependence of incident photon conversion efficiency (IPCE), using the intensity-calibrated Xe lamp with bandpass filters. Fig. 5 shows the result. IPCE reaches 0.45 at 0.6 VRHE and 420 nm, which matches the PEC photocurrent density at 0.56 VRHE by supposing a constant IPCE below 420 nm and multiplying the whole absorbed solar spectrum.
The present bulky crystallite BiVO4 thin film, with a superior mechanical robustness, exhibited high PEC performance and durability comparable to foregoing nanoporous and other types of O2-evolving undoped BiVO4 photoelectrodes.12,22,23 The robustness originates from the large BiVO4 grain size, which is inherited from the precursor BiO film, derived from the BiCxOy film deposited on ITO. The solid BiO calcination at 520 °C with diffusion of V from VO(acac)2 is the key process for the uniform formation of stoichiometric scheelite monoclinic BiVO4 without unbalanced agglomeration of the components. The purity and crystallinity of the light absorbing BiVO4 grains are probably the reasons for the good charge carrying and separating properties. Although the specific surface area might be smaller than that of porous-type materials, the impregnated CoOx and ALD NiO formed conformal rough surfaces, acting as a highly active cocatalyst for O2 evolution. The advantages of impregnated CoOx and ALD NiO cocatalysts were explained in detail in our previous report.8
Conclusions
We developed a new route for the synthesis of highly active, durable oxygen evolving, bulky crystalline BiVO4 film photoanodes on ITO glass. After modification with impregnated CoOx and ALD NiO, the BiVO4 electrode performs with a half-cell solar-to-hydrogen energy conversion efficiency of over 1.5% as an oxygen-evolving photoanode for water splitting under solar simulator AM 1.5G illumination. The high activity is maintained for 12 h at the operation potential of 0.6 and 0.9 VRHE with continuous and stoichiometric hydrogen and oxygen evolution. Mechanical strength is one of the superior properties owing to the firmly fixed BiVO4 crystallites on ITO substrates. The transparent characteristic of this electrode with respect to longer wavelength light in the solar spectrum further enables its use in tandem-type stand-alone water splitting devices assembled with hydrogen-evolving photocathodes made of narrow-bandgap p-type semiconductors.Acknowledgements
This work was financially supported by the Artificial Photosynthesis Project of the New Energy and Industrial Technology Development Organization (NEDO) and Ministry of Economy, Trade and Industry (METI). Authors thank Mrs Yoshie Nagatsuma, who enthusiastically carried out important experiments in this work.Notes and references
- M. Grätzel, Nature, 2001, 414, 338–344 CrossRef PubMed.
- S. W. Boettcher, J. M. Spurgeon, M. C. Putnam, E. L. Warren, D. B. Turner-Evans, M. D. Kelzenberg, J. R. Maiolo, H. A. Atwater and N. S. Lewis, Science, 2010, 327, 185–187 CrossRef CAS PubMed.
- Z. Wang, K. Teramura, S. Hosokawa and T. Tanaka, J. Mater. Chem. A, 2015, 3, 11313–11319 CAS.
- J. Zhao, T. Minegishi, L. Zhang, M. Zhong, M. Nakabayashi, G. J. Ma, T. Hisatomi, M. Katayama, S. Ikeda, N. Shibata, T. Yamada and K. Domen, Angew. Chem., Int. Ed., 2014, 53, 11808–11812 CrossRef CAS PubMed.
- M. Zhang, M. de Respinis and H. Frei, Nat. Chem., 2014, 6, 362–367 CrossRef CAS PubMed.
- M. W. Kanan and D. G. Nocera, Science, 2008, 321, 1072–1075 CrossRef CAS PubMed.
- L. Trotochaud, S. L. Young, J. K. Ranney and S. W. Boettcher, J. Am. Chem. Soc., 2014, 136, 6744–6753 CrossRef CAS PubMed.
- M. Zhong, T. Hisatomi, Y. Kuang, J. Zhao, M. Liu, A. Iwase, Q. Jia, H. Nishiyama, T. Minegishi, M. Nakabayashi, N. Shibata, R. Niishiro, C. Katayama, H. Shibano, M. Katayama, A. Kudo, T. Yamada and K. Domen, J. Am. Chem. Soc., 2015, 137, 5053–5060 CrossRef CAS PubMed.
- Y. Lin, Y. Xu, M. T. Mayer, Z. I. Simpson, G. Mcmahon, S. Zhou and D. Wang, J. Am. Chem. Soc., 2012, 134, 5508–5511 CrossRef CAS PubMed.
- F. F. Abdi, L. Han, A. H. M. Smets, M. Zeman, B. Dam and R. van de Krol, Nat. Commun., 2013, 4, 2195 Search PubMed.
- J. A. Seabold and K.-S. Choi, J. Am. Chem. Soc., 2012, 134, 2186–2192 CrossRef CAS PubMed.
- T. W. Kim and K.-S. Choi, Science, 2014, 343, 990–994 CrossRef CAS PubMed.
- A. Ziani, E. Nurlaela, D. S. Dhawale, D. A. Silva, E. Alarousu, O. F. Mohammed and K. Takanabe, Phys. Chem. Chem. Phys., 2015, 17, 2670–2677 RSC.
- F. F. Abdi, T. J. Savenije, M. M. May, B. Dam and R. van de Krol, J. Phys. Chem. Lett., 2013, 4, 2752–2757 CrossRef CAS.
- X. Shi, K. Zhang, J. Kwon, D. Y. Kim, J. K. Lee, S. H. Oh, J. K. Kim and J. H. Park, Nat. Commun., 2014, 5, 4775 CrossRef CAS PubMed.
- Y. Pihosh, I. Turkevych, K. Mawatari, J. Uemura, Y. Kazoe, S. Kosar, K. Makita, T. Sugaya, T. Matsui, D. Fujita, M. Tosa, M. Kondo and T. Kitamori, Sci. Rep., 2015, 5, 11141 CrossRef PubMed.
- M. Zhong, Y. Li, I. Yamada and J.-J. Delaunay, Nanoscale, 2012, 4, 1509–1514 RSC.
- M. Zhong, Y. Sato, M. Kurniawan, A. Apostoluk, B. Masenelli, E. Maeda, Y. Ikuhara and J.-J. Delaunay, Nanotechnology, 2012, 23, 495602 CrossRef PubMed.
- D. Kang, Y. Park, J. Hill and K.-S. Choi, J. Phys. Chem. Lett., 2014, 5, 2994–2999 CrossRef CAS PubMed.
- M. Zhong, Y. Ma, P. Oleynikov, K. Domen and J.-J. Delaunay, Energy Environ. Sci., 2014, 7, 1693–1699 CAS.
- S. Tokunaga, H. Kato and A. Kudo, Chem. Mater., 2001, 13, 4624–4628 CrossRef CAS.
- E. Alarcón-Lladó, L. Chen, M. Hettick, N. Mashouf, Y. Lin, A. Javey and J. W. Ager, Phys. Chem. Chem. Phys., 2014, 16, 1651–1657 RSC.
- L. Chen, F. M. Toma, J. K. Cooper, A. Lyon, Y. Lin, I. D. Sharp and J. W. Ager, ChemSusChem, 2015, 8, 1066–1071 CrossRef CAS PubMed.
- L. Chen, J. Yang, S. Klaus, L. J. Lee, R. Woods-Robinson, J. Ma, Y. Lum, J. K. Cooper, F. M. Toma, L. Wang, I. D. Sharp, A. T. Bell and J. W. Ager, J. Am. Chem. Soc., 2015, 137, 9595–9603 CrossRef CAS PubMed.
- A. A. Zavýalova, R. M. Imamov and Z. G. Pinsker, Kristallografiya, 1965, 10, 480 Search PubMed.
- M. Prekajski, A. Kremenović, B. Babić, M. Rosić, B. Matović, A. Radosavljević-Mihajlović and M. Radović, Mater. Lett., 2010, 64, 2247–2250 CrossRef CAS.
- K. Hirota, G. Komatsu, M. Yamashita, H. Takemura and O. Yamaguchi, Mater. Res. Bull., 1992, 27, 823–830 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |