Atomic defects during ordering transitions in LiNi0.5Mn1.5O4 and their relationship with electrochemical properties†
Abstract
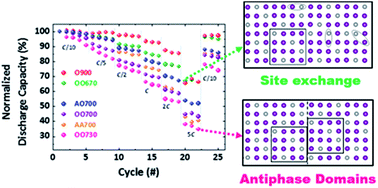
Decoupling the relevant parameters determining the electrochemical performance of spinel-type LiNi0.5Mn1.5O4 would contribute to promote its commercialization as cathode material for Li-ion batteries with high energy density. These parameters mainly comprise Ni/Mn ordering and non-stoichiometry, but their drivers and individual contribution to electrochemical performance remain to be fully ascertained. A series of samples annealed at different temperatures in the vicinity of an ordering transition have been thoroughly characterized by means of neutron powder diffraction to accurately establish composition–structure–property relationships in this material. The analysis revealed that deviations from a perfectly ordered crystal are possible through two different types of defects with significantly different effects on properties. These structural defects are in addition to previously described compositional defects, involving the creation of Mn3+ in the spinel lattice and Ni-rich rock salt secondary phases. Among the two types, the formation of antiphase boundaries is detrimental to transport, leading to poor rate performance of the electrode. In contrast, Ni/Mn mixing in an ordered framework can lead to behavior competitive with fully disordered samples, even at much lower Mn3+ contents that theoretically impart enhanced electronic conductivity. This work establishes design guidelines for fast transport in materials close to full stoichiometry, avoiding deleterious effects of rock salt impurities and Mn3+ dissolution.

 Please wait while we load your content...
Please wait while we load your content...