Synthesis of magnetic core–shell carbon dot@MFe2O4 (M = Mn, Zn and Cu) hybrid materials and their catalytic properties†
Abstract
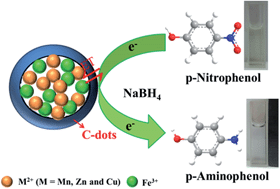
In this work, magnetic core–shell carbon dot@MFe2O4 (C-dot@MFe2O4) (M = Mn, Zn and Cu) hybrid materials are successfully prepared through a facile ultrasonic method. The resulting nanomaterials are well characterized using various techniques. It is indicated that the MFe2O4 microspheres are homogeneously dispersed and coated with low contrast continuous C-dot layers to form a core–shell structure. In addition, their catalytic activities are evaluated by measuring the reduction of p-nitrophenol (p-NP). The catalytic activity is found to strongly depend on the composition and morphology of the catalyst, and follows an order of C-dot@CuFe2O4 > CuFe2O4 > C-dot@MnFe2O4 > C-dot@ZnFe2O4 > MnFe2O4 > ZnFe2O4 > C-dots. C-dot@CuFe2O4 shows excellent activity with a rate constant of 8.05 × 104 min−1 g−1 which is superior to most reported catalysts. The good catalytic performance of C-dot@CuFe2O4 may be attributed to the specific characteristics of its nanostructure and the synergistic effect between CuFe2O4 and the C-dots. The effects of the substituent and reducing agent are investigated. Furthermore, the catalyst can be easily recovered using a magnetic field and shows good stability; it can be reused for five successive experiments with a conversion efficiency of more than 95%. This novel catalyst also exhibits excellent catalytic performance for the degradation of other organic dyes, such as methylene blue (MB) and rhodamine B (RhB), and can be used for the purification of contaminated water. These results indicate that C-dot@CuFe2O4 is an efficient catalyst for environmental wastewater treatment.

 Please wait while we load your content...
Please wait while we load your content...