Open Access Article
Open Access ArticleHigh performance PEDOT/lignin biopolymer composites for electrochemical supercapacitors†
F. N.
Ajjan‡
a,
N.
Casado‡
b,
T.
Rębiś
a,
A.
Elfwing
a,
N.
Solin
a,
D.
Mecerreyes
bc and
O.
Inganäs
*a
aBiomolecular and Organic Electronics IFM, Linköping University, S-581 83, Linköping, Sweden. E-mail: fataj@ifm.liu.se.com; oling@ifm.liu.se
bPOLYMAT, University of the Basque Country UPV/EHU, Joxe Mari Korta Center, Avda. Tolosa 72, 20018 Donostia-San Sebastian, Spain. E-mail: david.mecerreyes@ehu.es
cIKERBASQUE, Basque Foundation for Science, E-48011, Bilbao, Spain
First published on 4th January 2016
Abstract
Developing sustainable organic electrode materials for energy storage applications is an urgent task. We present a promising candidate based on the use of lignin, the second most abundant biopolymer in nature. This polymer is combined with a conducting polymer, where lignin as a polyanion can behave both as a dopant and surfactant. The synthesis of PEDOT/Lig biocomposites by both oxidative chemical and electrochemical polymerization of EDOT in the presence of lignin sulfonate is presented. The characterization of PEDOT/Lig was performed by UV-Vis-NIR spectroscopy, FTIR infrared spectroscopy, thermogravimetric analysis, scanning electron microscopy, cyclic voltammetry and galvanostatic charge–discharge. PEDOT doped with lignin doubles the specific capacitance (170.4 F g−1) compared to reference PEDOT electrodes (80.4 F g−1). The enhanced energy storage performance is a consequence of the additional pseudocapacitance generated by the quinone moieties in lignin, which give rise to faradaic reactions. Furthermore PEDOT/Lig is a highly stable biocomposite, retaining about 83% of its electroactivity after 1000 charge/discharge cycles. These results illustrate that the redox doping strategy is a facile and straightforward approach to improve the electroactive performance of PEDOT.
Introduction
The development of energy storage devices based on sustainable bio-inspired components is at present heavily researched.1 Lignin, the second most abundant biopolymer on earth, contains a large variety of redox-active phenolic type functional groups that enable storage of charge.2 The development of charge storage devices based on lignin would be a large step towards the development of sustainable devices. Lignin is also a side product in the processing of wood for paper, and is mainly burnt to produce process heat. It is used as a surfactant in the processing of cement, but such uses only cover a small fraction of the waste product use. It is therefore a cheap and scalable material. However, lignin in itself is an insulating material. Accordingly, it is necessary to incorporate lignin into a matrix consisting of a conducting material, in order to allow charge transport.3,4 The conductor may then transport the charge to the lignin moiety where the charge can be stored in a reversible reduction–oxidation conversion between the hydroquinone and quinone forms formed from monolignols. The first example of this approach utilized a combination of lignin and polypyrrole.5 In addition, various combinations of lignin and conductors such as reduced graphene oxide6 or carbon nanotubes7 have been investigated. The use of conducting polymers is attractive as their properties can be modified by variations in the polymer structure.Poly(3,4-ethylenedioxythiophene) (PEDOT) is a highly conductive, electrochemically and thermally stable conducting polymer.8 The polymerization of the monomer (EDOT) is facile; however, the low solubility of PEDOT makes the processing of the material challenging. This drawback can be overcome by polymerizing EDOT in the presence of a water-soluble polyelectrolyte.9–13 Most commonly, polystyrenesulfonate (PSS) is employed, resulting in a PEDOT:PSS dispersion in water, where PSS plays the dual role of a dopant and dispersing agent. Supercapacitor applications have been studied using these types of materials since some time.14–16 Lignin can be processed into the so-called lignosulfonate, containing sulfonate groups that, in a similar manner to the sulfonate groups in PSS, could enable lignosulfonate to act both as a dopant and dispersing agent.17 It could thus be considered that lignosulfonate could disperse EDOT in aqueous solution and upon EDOT polymerization a PEDOT/lignosulfonate composite would form. Such PEDOT/lignin (PEDOT/Lig) composites can in principle be synthesized by two different approaches: chemical polymerization or electrochemical polymerization. Chemical polymerization allows bulk production of polymeric dispersions, which makes chemical polymerization the method of choice for commercial applications. On the other hand, electrochemical polymerization is carried out by the galvanostatic technique, which allows the deposition of homogeneous films onto the working electrode with a well-controlled thickness and morphology.18
The aim of the present study is to investigate electrochemically and chemically synthesized PEDOT/Lig composites and compare their redox behavior, electrochemical stability and surface morphology.
Results and discussion
PEDOT/lignin synthesis
Scheme 1 shows the synthetic pathways of PEDOT/Lig composites. The PEDOT/Lig composites were synthesized both using chemical and electrochemical polymerization. In both cases the resulting biomaterial is an ionic complex composite where both PEDOT and lignin sulfonates are closely combined similar to interpenetrating polymer networks. | ||
| Scheme 1 (a) Chemical oxidative polymerization and (b) electrochemical polymerization of the PEDOT/Lig composite. | ||
PEDOT/Lig composites were synthesized via chemical oxidative polymerization of EDOT in the presence of lignin, by using an iron redox agent in a catalytic amount and a primary oxidant.
When PEDOT is synthesized through oxidative polymerization with oxidizing agents, such as persulfate or iron(III) salts, the typical molecular weight of PEDOT is not higher than 1000 to 2500 Da (6–18 repeating units); therefore, we assume that the molecular weight of PEDOT molecules in the composites is in the same range.19 The synthetic strategy for the preparation of PEDOT/Lig composites was carried out containing different EDOT/lignin mass ratios (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 7
1; 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4; 3
4; 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; 5
2; 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4; 2
4; 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; 1
3; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). The final PEDOT/lignin ratio was calculated from lignin quantification by UV-vis.20 First, a lignin calibration curve was obtained by measuring the absorbance at 202 nm of different lignin standards with concentrations between 0.0025 and 0.20 g L−1, as shown in Fig. S1.† Then, the absorbance of PEDOT/lignin dispersions was measured and the lignin concentration on the dispersion was calculated with the calibration curve. The calculated final ratio of the composites and the relation between the feed and product ratios are shown in Fig. 1b, the product ratio is quite similar to the initial feed.
3). The final PEDOT/lignin ratio was calculated from lignin quantification by UV-vis.20 First, a lignin calibration curve was obtained by measuring the absorbance at 202 nm of different lignin standards with concentrations between 0.0025 and 0.20 g L−1, as shown in Fig. S1.† Then, the absorbance of PEDOT/lignin dispersions was measured and the lignin concentration on the dispersion was calculated with the calibration curve. The calculated final ratio of the composites and the relation between the feed and product ratios are shown in Fig. 1b, the product ratio is quite similar to the initial feed.
The electrochemical polymerization of EDOT in the presence of lignin was carried out under galvanostatic conditions. Polymerizations were carried out at a current density of 0.25 mA cm−2 for 300 s, 600 s and 900 s in a solution consisting of 10 mM EDOT and 1 mg mL−1 of lignin in a 0.1 M HClO4/water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile (9
acetonitrile (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixed solvent, as represented in Scheme 1b. Depending on the polymerization time, composite films with increasing thicknesses were obtained on the electrodes.
1) mixed solvent, as represented in Scheme 1b. Depending on the polymerization time, composite films with increasing thicknesses were obtained on the electrodes.
The formation of PEDOT/lignosulfonate composites might be understood as a rearrangement and clustering process during the polymerization synthesis giving rise to easier charge transport. Moreover, as sulfonates in lignin carry a negative charge, they are expected to act as a charge balancing counter-ion to the positively doped PEDOT chains facilitating the association of PEDOT chains with lignin via electrostatic interaction during the polymerization.
Electrochemical polymerization offers some advantages over chemical polymerization such as the absence of a catalyst and control of the thickness and morphology of the deposited film. Moreover, the quality of the electropolymerized PEDOT film layer is usually higher compared to that obtained after casting the PEDOT dispersions. On the other hand, the chemical polymerization has other clear advantages since it allows controlling the ratio between PEDOT and lignin in the final composites. Furthermore, it allows the production of PEDOT/Lig composites on a large scale compared to electrochemical routes. In the next sections, we will characterize and compare the different PEDOT/Lig composites obtained chemically and electrochemically.
Characterization of chemically polymerized PEDOT/lignin composites
Dark blue aqueous dispersions having different PEDOT/Lig ratios were synthesized by chemical oxidative polymerization. The UV/vis spectra of the resulting PEDOT/Lig dispersions (as well as, for reference, the spectra of lignin and EDOT) are shown in Fig. 1. The lignin-sulfonate spectrum has two distinctive peaks. The peak at 202 nm is assigned to the conjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of the aromatic structure and another one at 280 nm corresponds to p-substituents in phenolic groups depending on the different monolignol units (coumaryl, coniferyl and sinapyl) in lignin due to their electron donating character.21,22 The absorption spectrum of PEDOT depends on the redox state of the polymer. Neutral PEDOT has an absorption band at 600 nm, caused by π–π* transitions. On the other hand, the absorption spectra of doped PEDOT have absorption maxima located at 800 nm and ∼1100 nm which are attributed to polaronic and bipolaronic states.23 The absorbance spectra of PEDOT/Lig dispersions display transitions typical of both lignin (202, 283 nm) and doped PEDOT (800 nm, <1000 nm). The appearance of UV-vis spectra thus confirms that EDOT has been successfully polymerized in the presence of lignosulfonate, obtaining an oxidized PEDOT which is stabilized by lignosulfonate.
C of the aromatic structure and another one at 280 nm corresponds to p-substituents in phenolic groups depending on the different monolignol units (coumaryl, coniferyl and sinapyl) in lignin due to their electron donating character.21,22 The absorption spectrum of PEDOT depends on the redox state of the polymer. Neutral PEDOT has an absorption band at 600 nm, caused by π–π* transitions. On the other hand, the absorption spectra of doped PEDOT have absorption maxima located at 800 nm and ∼1100 nm which are attributed to polaronic and bipolaronic states.23 The absorbance spectra of PEDOT/Lig dispersions display transitions typical of both lignin (202, 283 nm) and doped PEDOT (800 nm, <1000 nm). The appearance of UV-vis spectra thus confirms that EDOT has been successfully polymerized in the presence of lignosulfonate, obtaining an oxidized PEDOT which is stabilized by lignosulfonate.
The EDOT monomer shows a maximum absorption at 260 nm, which shifts to higher wavelengths during the polymerization, as the π system conjugation is extended as the polymer is formed.24 Since EDOT does not show absorption at 202 nm, all PEDOT/Lig spectra in Fig. 1 have been normalized with respect to the lignin absorption peak at 202 nm. Therefore, the absorption intensity of the bands between 600 and 1300 nm reveals the relative proportion of PEDOT to lignin in the dispersions. Inspection of the spectra accordingly allows us to confirm that polymerization carried out with a higher relative ratio of EDOT to lignosulfonate results in an increase of PEDOT band intensity.
The thermal behavior of PEDOT/Lig composites was investigated by thermogravimetric analysis (TGA), performed under nitrogen atmosphere, measuring the weight loss as a function of temperature (Fig. 2). The signal of lignin alone is considered as a control in order to compare the different PEDOT/Lig composites.12,25 TGA curves show that all the PEDOT/Lig composites are stable up to 250 °C, similar to the control lignin. The most noticeable degradation occurs between 300 °C and 400 °C, and is attributed to the decomposition of PEDOT. The control lignin shows residual traces of 52% of the total mass; however the residual traces decrease as the content of PEDOT increases. To sum up, the TGA results are consistent with the final PEDOT/lignin composition.
FT-infrared (FTIR) spectra of lignin and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 PEDOT/Lig composites are shown in Fig. 3. The lignin spectrum shows peaks at 1600 and 1505 cm−1 attributed to the vibrations of the aromatic ring, and bands at 1460 and 1422 cm−1 related to the aromatic ring vibrations combined with methyl and methylene C–H deformations. A broad peak is observed at 1215 cm−1 due to C–C and C–O stretching vibrations merged aromatic ring stretching modes and a strong peak at 1040 cm−1 is attributed to C–O–C stretching vibrations. These results are in agreement with the previously reported lignin spectra.26 On the other hand, all PEDOT/Lig composites show similar spectra exhibiting bands related to both PEDOT and lignin structures. PEDOT/Lig composites show peaks at 1500, 1380 and 1340 cm−1 corresponding to aromatic ring vibrations in lignin and the thiophene ring. Bands at 1197, 1089, 1055 cm−1 are attributed to C–O–C stretching modes in the composites, and the peak at 838 cm−1 is related to C–S stretching vibration of the thiophene ring. These results are consistent with electrochemically and chemically synthesized PEDOT.27,28
2 PEDOT/Lig composites are shown in Fig. 3. The lignin spectrum shows peaks at 1600 and 1505 cm−1 attributed to the vibrations of the aromatic ring, and bands at 1460 and 1422 cm−1 related to the aromatic ring vibrations combined with methyl and methylene C–H deformations. A broad peak is observed at 1215 cm−1 due to C–C and C–O stretching vibrations merged aromatic ring stretching modes and a strong peak at 1040 cm−1 is attributed to C–O–C stretching vibrations. These results are in agreement with the previously reported lignin spectra.26 On the other hand, all PEDOT/Lig composites show similar spectra exhibiting bands related to both PEDOT and lignin structures. PEDOT/Lig composites show peaks at 1500, 1380 and 1340 cm−1 corresponding to aromatic ring vibrations in lignin and the thiophene ring. Bands at 1197, 1089, 1055 cm−1 are attributed to C–O–C stretching modes in the composites, and the peak at 838 cm−1 is related to C–S stretching vibration of the thiophene ring. These results are consistent with electrochemically and chemically synthesized PEDOT.27,28
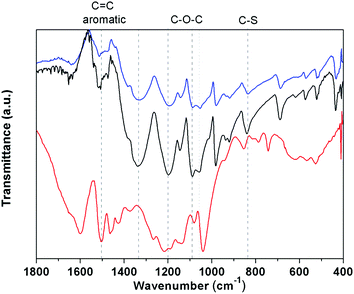 | ||
Fig. 3 FTIR spectra of lignin (red line) and PEDOT/Lig composites of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (black line) and 3 1 (black line) and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (blue line) mass ratios. 2 (blue line) mass ratios. | ||
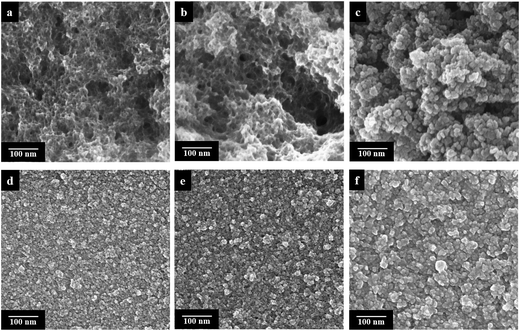
The electrochemical performance of an electrode is largely dependent on the morphology and surface area of the electrode material. Therefore, Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) were used to investigate the surface morphology of both chemically and electrochemically polymerized PEDOT/Lig composites (Fig. 4). SEM images of the chemically polymerized biocomposites of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 7
2 and 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 mass ratios exhibit a granular-spongy morphology (Fig. 4). The PEDOT/Lig composite of 6
4 mass ratios exhibit a granular-spongy morphology (Fig. 4). The PEDOT/Lig composite of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio shows the formation of aggregates because of the existence of a higher content of PEDOT. For the three electropolymerized PEDOT/Lig composites at different deposition times (300 s, 600 s and 900 s), a more compact, dense structure with a slightly granular surface is observed with increasing grain size with the deposition time. An analogous effect in surface morphology is also observed in the electrochemically deposited PEDOT film (Fig. S7†). Comparing the electrochemically polymerized composites with the one chemically polymerized at the same EDOT/lignin ratio (3
1 mass ratio shows the formation of aggregates because of the existence of a higher content of PEDOT. For the three electropolymerized PEDOT/Lig composites at different deposition times (300 s, 600 s and 900 s), a more compact, dense structure with a slightly granular surface is observed with increasing grain size with the deposition time. An analogous effect in surface morphology is also observed in the electrochemically deposited PEDOT film (Fig. S7†). Comparing the electrochemically polymerized composites with the one chemically polymerized at the same EDOT/lignin ratio (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, Fig. 4a), it can be seen that the electrochemical polymerization gives rise to a more homogeneous surface than the chemically polymerized one.
2, Fig. 4a), it can be seen that the electrochemical polymerization gives rise to a more homogeneous surface than the chemically polymerized one.
Fig. S8† shows TEM images of the chemically polymerized PEDOT/lignin composites with (a) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; (b) 7
2; (b) 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4; (c) 6
4; (c) 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EDOT
1 EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lignin mass ratios. TEM images reveal that PEDOT/lignin composites form aggregates of various sizes, the majority being 200–400 nm size. It seems that these aggregates consist of various tangles which have a PEDOT rich inner part and a lignin rich outer part.
lignin mass ratios. TEM images reveal that PEDOT/lignin composites form aggregates of various sizes, the majority being 200–400 nm size. It seems that these aggregates consist of various tangles which have a PEDOT rich inner part and a lignin rich outer part.
We can therefore hypothesize that the influence of a larger content of lignin on the chemically polymerized biocomposites leads to a greater roughness, leading to a larger surface area which provides more accessible electroactive ions during electrochemical processes.
Electrochemical characterization of PEDOT/lignin composites
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), showed no charge transport. This is probably due to the insulating character of lignin, which dominates the oxidative polymerization giving a non-conducting composite. The stored charge of quinones in lignin was estimated considering the integration area of the quinone peaks and the total charge which is stored in each PEDOT/Lig composite (Fig. 5b), achieving an optimum relationship for the 3
3), showed no charge transport. This is probably due to the insulating character of lignin, which dominates the oxidative polymerization giving a non-conducting composite. The stored charge of quinones in lignin was estimated considering the integration area of the quinone peaks and the total charge which is stored in each PEDOT/Lig composite (Fig. 5b), achieving an optimum relationship for the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (40% of lignin), 7
2 (40% of lignin), 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (36% of lignin) and 5
4 (36% of lignin) and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (44% of lignin) ratio of PEDOT/Lig composites. The charge storage behavior of 3
4 (44% of lignin) ratio of PEDOT/Lig composites. The charge storage behavior of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 7
2 and 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio of PEDOT/Lig composites exhibit comparable redox activity showing well defined redox peaks with higher capacity over all biocomposite electrode materials. Nevertheless, it is worth noticing the contribution of redox quinone activity in lignin for 5
4 ratio of PEDOT/Lig composites exhibit comparable redox activity showing well defined redox peaks with higher capacity over all biocomposite electrode materials. Nevertheless, it is worth noticing the contribution of redox quinone activity in lignin for 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio. As the content of PEDOT is higher than the content of lignin for the 6
4 ratio. As the content of PEDOT is higher than the content of lignin for the 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, the hydroquinone/quinone redox peaks of lignin decrease as the PEDOT region increases. The increase of PEDOT content clearly shows typical capacitor performance with improved charge propagation.
1 ratio, the hydroquinone/quinone redox peaks of lignin decrease as the PEDOT region increases. The increase of PEDOT content clearly shows typical capacitor performance with improved charge propagation.
Given the electrochemical properties of PEDOT/Lig, the feed mass ratios 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 7
2, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 6
4 and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were chosen in order to analyze the redox activities of the composite material. Fig. 6 shows the galvanostatic charge/discharge curves of PEDOT/Lig composites obtained at a current density of 1 A g−1 within the potential window of 0.1 to 0.7 V. It can be clearly seen that the 3
1 were chosen in order to analyze the redox activities of the composite material. Fig. 6 shows the galvanostatic charge/discharge curves of PEDOT/Lig composites obtained at a current density of 1 A g−1 within the potential window of 0.1 to 0.7 V. It can be clearly seen that the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 7
2 and 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 PEDOT/Lig mass ratios have very similar specific charge values of 28.0 mA h g−1 and 27.6 mA h g−1, respectively. In contrast the 6
4 PEDOT/Lig mass ratios have very similar specific charge values of 28.0 mA h g−1 and 27.6 mA h g−1, respectively. In contrast the 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio has a specific charge of 18.5 mA h g−1. The charge storage capacity can thus be modified by varying the PEDOT/Lig ratio, and at the optimum dopant content of lignin, the charge storage capacity of the conducting polymer/biopolymer composite is improved up to 40% compared to the 6
1 ratio has a specific charge of 18.5 mA h g−1. The charge storage capacity can thus be modified by varying the PEDOT/Lig ratio, and at the optimum dopant content of lignin, the charge storage capacity of the conducting polymer/biopolymer composite is improved up to 40% compared to the 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PEDOT/Lig ratio. Moreover the discharge curves show a plateau between 0.55 V and 0.35 V, which can be explained by faradaic reactions from quinones. Furthermore it is worth noting how the charge is stored following the capacitive behavior of PEDOT. The discharge properties of each system at different current densities were also studied (Fig. S3†). As expected, the charge stored at 1 A g−1 is larger than that at higher current densities, although for PEDOT/Lig mass ratios of 6
1 PEDOT/Lig ratio. Moreover the discharge curves show a plateau between 0.55 V and 0.35 V, which can be explained by faradaic reactions from quinones. Furthermore it is worth noting how the charge is stored following the capacitive behavior of PEDOT. The discharge properties of each system at different current densities were also studied (Fig. S3†). As expected, the charge stored at 1 A g−1 is larger than that at higher current densities, although for PEDOT/Lig mass ratios of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7
1, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 3
4 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, a retention of 95%, 82% and 85% is observed, respectively, at 16 A g−1 suggesting a good rate capability of the biocomposite material.
2, a retention of 95%, 82% and 85% is observed, respectively, at 16 A g−1 suggesting a good rate capability of the biocomposite material.
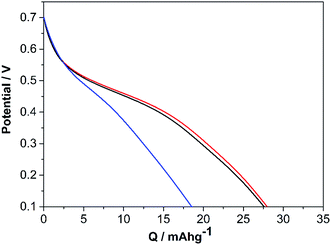 | ||
Fig. 6 Galvanostatic discharge curves of the PEDOT/Lig composite polymerized at different mass ratios, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (blue line); 7 1 (blue line); 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (black line); 3 4 (black line); 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (red line); at a current density of 1 A g−1. 2 (red line); at a current density of 1 A g−1. | ||
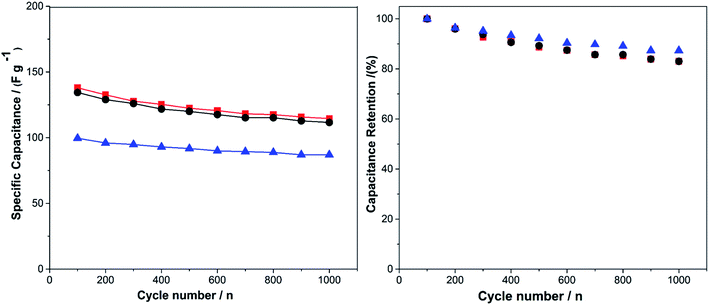
Cycling stability is one of the most important parameters for the performance of a supercapacitor electrode. For this purpose, the charge–discharge cycle life of PEDOT/Lig composites was tested applying potentials from 0.1 to 0.7 V for up to 1000 cycles (Fig. 7). Upon cycling, the PEDOT/Lig composites showed a decrease in the initial capacitance, which was less than 20% after 1000 cycles. The main loss occurred during the first 100 cycles. After that the specific capacitance values of the original capacitor were retained by more than 90%. This loss of capacity can be explained by degradation of the quinone groups in lignin, resulting in a loss of redox activity. In contrast, PEDOT shows no variation in terms of electrochemical stability after 1000 cycles (Fig. S6A†). The PEDOT/Lig composites for the mass ratios of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7
1, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 3
4 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 show specific capacitances of 87 F g−1, 112 F g−1 and 115 F g−1, respectively, at a discharge current of 8 A g−1 after 1000 cycles (Table 1).
2 show specific capacitances of 87 F g−1, 112 F g−1 and 115 F g−1, respectively, at a discharge current of 8 A g−1 after 1000 cycles (Table 1).
| Sample | Q (mA h g−1) | C (F g−1) | Capacity retention after 1000 cycles (%) | Columbic efficiency (%) |
|---|---|---|---|---|
| PEDOT | 14.0 | 80.4 | 96 | 92 |
PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lignin 300 s lignin 300 s |
33.4 | 170.4 | 80 | 91 |
PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lignin 600 s lignin 600 s |
34.1 | 170.4 | 83 | 90 |
PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lignin 900 s lignin 900 s |
31.9 | 161.4 | 82 | 92 |
PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lignin 3 lignin 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
28.0 | 115.0 | 83 | 96 |
PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lignin 7 lignin 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
27.6 | 112.0 | 83 | 95 |
PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lignin 6 lignin 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
18.5 | 87.0 | 87 | 96 |
The charge storage from lignin is higher for the electrochemically synthesized PEDOT/Lig composite giving rise to 50% of the total charge, compared to 24% of charge capacity stored for the chemically polymerized PEDOT/Lig composite in the optimum mass ratio.
The cycling stability of the electrochemically polymerized composites was tested applying potentials from 0.1 to 0.7 V for up to 1000 cycles (similar to the previous measurements for the chemically polymerized composites). PEDOT without lignin demonstrates a specific capacitance of 80.4 F g−1 and only 4% loss of total capacitance after 1000 cycles. Regarding PEDOT/Lig composites the obtained specific capacitances were 170.4 F g−1, 170.4 F g−1 and 161.4 F g−1 for 300 s, 600 s and 900 s, respectively. The capacitive retention at 8 A g−1 current density was approximately 82% for all PEDOT/Lig composites (Fig. 9). As mentioned before, the decrease of capacity in PEDOT/Lig composites is due to the lower redox activity displayed by lignin as the number of cycles increases (Table 1).
Self-discharge
Self-discharge is one of the main problems of conducting polymers and energy storage devices made out of them.29,30Fig. 10 shows self-discharge with open circuit potential in a three electrode system, decaying to 0.33 V for PEDOT and ca. 0.40 V for the majority of PEDOT/Lig composites after 16 h. PEDOT without lignin showed the most pronounced self-discharge. The self discharge process for the electrochemically polymerized PEDOT/Lig composites exhibits a slight decrease as the electrodeposition time increases. However, when a higher deposition time is used, the observed self-discharge behavior is faster. Concerning the chemically polymerized PEDOT/Lig composites, the maximum self-discharge is observed for the biocomposite with a larger PEDOT fraction. Consequently PEDOT/Lig composites alleviate the self-discharge problems of conducting polymer electrodes. | ||
| Fig. 10 Self-discharge behaviour of (a) PEDOT/Lig composites chemically polymerized and (b) PEDOT and PEDOT/Lig composites electrochemically polymerized. | ||
Influence of pH
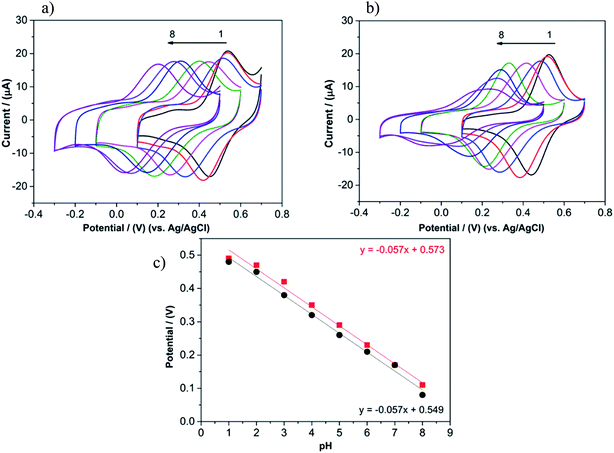
The electrochemistry of quinone moieties is pH dependent due to the H+ exchange during redox reactions. Hence it is possible to confirm the reversibility of hydroquinone/quinone conversion during the redox process by testing it within a broad pH range. PEDOT/Lig composites, whether obtained by chemical (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) or electrochemical polymerisation (300 s) exhibit comparable redox activity across different pHs (over the pH range 1.0–8.0) as measured by cyclic voltammetry. It was observed that in both composites, the peak potentials are shifted negatively with the increase of pH. The linear relationship between the peak potentials and the pH values has a slope of −0.057 V per pH for both composites, very close to the theoretical Nernstian value.7 Taken together, these data indicate that the redox behavior of PEDOT/Lig electrodes is governed by two-electron two-proton processes that oxidize hydroquinones into quinones.
2) or electrochemical polymerisation (300 s) exhibit comparable redox activity across different pHs (over the pH range 1.0–8.0) as measured by cyclic voltammetry. It was observed that in both composites, the peak potentials are shifted negatively with the increase of pH. The linear relationship between the peak potentials and the pH values has a slope of −0.057 V per pH for both composites, very close to the theoretical Nernstian value.7 Taken together, these data indicate that the redox behavior of PEDOT/Lig electrodes is governed by two-electron two-proton processes that oxidize hydroquinones into quinones.
The chemically polymerized PEDOT/Lig composite does not present a significant decrease of peak current, but more reversible behavior with increase of pH. However for the electrochemically polymerized composite there is a noticeable decrease of peak amplitude at a higher pH. This is most likely due to kinetic limitation of electron transfer which is observed by a large peak separation (cathodic peak potential (Epc) of 0.27 V at pH 8 and 0.085 V at pH 1), or possible hampered diffusion of H+ through the film31 (Fig. 11). This is in agreement with SEM images, where a denser and flatter morphology was observed. On the other hand, the observed decrease of electroactivity at neutral pH, is not because of hydrolysis, as no degradation was detected when the PEDOT/Lig composite electrode was returned to acidic media.
Comparative analysis between lignin composite materials
Recently, steps have been taken to study energy storage applications by incorporating lignin into a biocomposite within different conducting matrices. For instance Park et al.6 were able to reduce graphene oxide by confining lignin nanocrystals on the surface. The electroactive biomaterial exhibits a capacitance of 432 F g−1 with 96% retention after 3000 cycles. Milczarek et al.7 studied multiwalled carbon nanotubes functionalized with lignin obtaining 177 F g−1 at 2.5 A g−1, reaching stable capacitance up to 500 cycles. Moreover we previously studied lignin combined with polypyrrole achieving 75 mA h g−1.5 Therefore polypyrrole/lignin (PPy/Lig) and PEDOT/Lig can be considered analogous electrode materials. The galvanostatic electrochemical polymerization of PPy/Lig and PEDOT/Lig is performed under similar conditions (Fig. S9†). The obtained masses of both biopolymer composites were comparable (51 μg and 45 μg for PPy/Lig and PEDOT/Lig, respectively), suggesting that the efficiency during the PEDOT/Lig electropolymerization is lower than that for the PPy/Lig synthesis. This effect is because the oxidation potential for PEDOT/Lig is higher than that for PPy/Lig, leading to the simultaneous oxidation of lignin. Thus the quinones in lignin are generated during the electrochemical synthesis of PEDOT/Lig biocomposites. On the other hand, in order to generate quinones within PPy/Lig it is necessary to activate the electrode materials by scanning up to 0.7 V. Comparing the performance of the two different biopolymer composites, we noted that the charge capacity is lower in the PEDOT/Lig composite than that in the PPy/Lig composite. This behavior can be attributed to different factors such as the molecular weight of the monomer of the conducting polymer (pyrrole and EDOT, respectively), the interaction between quinone moieties in lignin and the conducting polymers and the amount of lignin incorporated within the biocomposite material. However the resulting stored charge from lignin in both PPy/Lig and PEDOT/Lig composites was 50% showing a similar interaction between quinone groups present in lignin and the conducting polymers. Taking into account that the mass of incorporated lignin within both composites through electrochemical synthesis is hard to assess, which is the ideal charge capacity that could be stored in this type of hybrid material.Nonetheless the PEDOT/Lig composite shows superior stability performance compared to the PPy/Lig composite. The PEDOT/Lig composite retains 82% of the initial capacity after 1000 cycles maintaining the quinone redox electroactivity in contrast to the PPy/Lig composite which shows a decay of electroactivity with increasing numbers of cycles due to the degradation of polypyrrole within the composite.26
Experimental
Chemicals and reagents
3,4-Ethylenedioxythiophene (EDOT, 99%, Acros organics), a lignin derivative in the form of lignosulfonates (LC30, Mw = 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400, MeadWestvaco), sodium persulfate (Na2S2O8), iron(III)chloride (FeCl3), acetonitrile (MeCN) and perchloric acid (HClO4) (Sigma-Aldrich) were used as received. The aqueous solutions were prepared with ultrapure deionized water (Millipore).
400, MeadWestvaco), sodium persulfate (Na2S2O8), iron(III)chloride (FeCl3), acetonitrile (MeCN) and perchloric acid (HClO4) (Sigma-Aldrich) were used as received. The aqueous solutions were prepared with ultrapure deionized water (Millipore).
Chemical synthesis of PEDOT/lignin composites
The polymerization of EDOT/lignin was accomplished by dropwise addition of an aqueous mixture of Na2S2O8 (1.5 eq. EDOT) and a catalytic amount of FeCl3 as an oxidant to a solution of EDOT and lignin in water, yielding a biocomposite material. The oxidative polymerization was carried out under mechanical stirring at r.t. for 8 h and the concentration of the solution was kept constant at 1% (w/v). Instantaneously upon addition of the oxidant mixture, the EDOT/lignin solution turned from brownish to deep blue colored solution. Finally, the obtained PEDOT/Lig dispersions were dialyzed with deionized water for 48 hours using a 1000 g mol−1 cutoff membrane and freeze-dried, yielding PEDOT/Lig as a dark bluish powder. The overall yield of the polymerization was 85%.Electrochemical synthesis of PEDOT/lignin composites
The electrochemical polymerization was carried out under galvanostatic conditions onto the prior polished glassy carbon (GC) electrodes (0.07 cm2 geometrical area), by applying a constant current density of 0.25 mA cm−2 for 300 s, 600 s and 900 s (75 mC cm−2, 150 mC cm−2 and 225 mC cm−2 total charge, respectively, to the deposition times) considering the EDOT/lignin initial ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
Electrodes preparation
Regarding the chemically polymerized biocomposite material, 200 μl of homogeneous mixtures of the different chemically polymerized PEDOT/Lig materials were deposited by drop-casting onto glass, dried in the oven and finally weighed using a balance (Sartorius BP 210D). Afterwards, 1 μl of homogeneous dispersion was deposited by drop-casting onto the prior polished GC electrodes. The deposited mass of the different PEDOT/Lig solutions were 8.6 μg (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 10.9 μg (7
1), 10.9 μg (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), 7.9 μg (3
4), 7.9 μg (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), 8.0 μg (5
2), 8.0 μg (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), 7.5 μg (2
4), 7.5 μg (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), 5.5 μg (1
3), 5.5 μg (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), respectively.
3), respectively.
Composite characterization
| C = (IΔt)/(mΔE) |
The quartz crystal microbalance studies were carried out using a Q-Sense E4 and gold deposited quartz crystals (1 cm2 diameter). The frequency change was monitored during the deposition of the material, and analysed using the Sauerbrey equation to deduce the masses of these stiff materials. All the measurements were made in a 0.1 M HClO4/water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile (9
acetonitrile (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixed solvent as the electrolyte.
1) mixed solvent as the electrolyte.
The calculation of the capacitance was based on the mass of the whole PEDOT/lignin composite obtained by using a quartz crystal microbalance and balance weight determination depending on the type of polymerization of the biocomposite materials. Regarding PEDOT without lignin, the calculation of capacity is based on only the mass of PEDOT as obtained from quartz crystal microbalance measurements.
Conclusions
In this study, we have investigated two synthetic routes, chemical and electrochemical polymerization of PEDOT with lignin. A detailed comparative study of the aqueous solution behavior of PEDOT/Lig composite was presented, which demonstrates the importance of the addition of the hydroquinone/quinone redox couple within the chemical structure of lignin to accomplish a significant improvement in charge storage performance.The highest specific capacitance obtained for the electrochemically polymerized biocomposite was 170.4 F g−1, showing more than twice the specific capacitance of the standard PEDOT polymer (80.4 F g−1). For the chemically polymerized PEDOT/Lig composite, the highest specific capacitance was found to be lower, 115 F g−1 than for the electrochemically polymerized electrode material. All PEDOT/Lig composites retained 83% of capacity after 1000 cycles and exhibit more than 90% of coulombic efficiency. The evidence of higher stability values for PEDOT/Lig composites reinforces the idea that an important synergic effect exists between both polymers compared to the previous study of polypyrrole/lignin composites, where strong degradation was observed upon cycling. Hence PEDOT/Lig composites exhibit higher capacitive performance which is advantageous over the performance of PEDOT without lignin for further applications.
These encouraging findings open up the possibility of scaling up the PEDOT/Lig production thanks to the chemical synthesis, satisfying the requirements (inexpensiveness, environmental friendliness and easy processability) for potential charge storage materials in supercapacitors and supercabatteries.
Acknowledgements
This work was supported by the Power Papers project, and a Wallenberg Scholar grant to Olle Inganäs, both from the Knut and Alice Wallenberg foundation, by Marie Curie network Renaissance (NA) and European Research Council by Starting Grant Innovative Polymers for Energy Storage (iPes) 306250 (DM). Nerea Casado thanks the Basque Government for the predoctoral fellowship received to carry out this work.Notes and references
- L. Zhang, Z. Liu, G. Cui and L. Chen, Prog. Polym. Sci., 2015, 43, 136–164 CrossRef CAS
.
- G. Milczarek, Langmuir, 2009, 25, 10345–10353 CrossRef CAS PubMed
.
- B. Huskinson, M. P. Marshak, C. Suh, S. Er, M. R. Gerhardt, C. J. Galvin, X. Chen, A. Aspuru-Guzik, R. G. Gordon and M. J. Aziz, Nature, 2014, 505, 195–198 CrossRef CAS PubMed
.
- Y. J. Kim, W. Wu, S.-E. Chun, J. F. Whitacre and C. J. Bettinger, Adv. Mater., 2014, 26, 6572–6579 CrossRef CAS PubMed
.
- G. Milczarek and O. Inganäs, Science, 2012, 335, 1468–1471 CrossRef CAS PubMed
.
- S.-K. Kim, Y. K. Kim, H. Lee, S. B. Lee and H. S. Park, ChemSusChem, 2014, 7, 1094–1101 CrossRef CAS
.
- G. Milczarek and M. Nowicki, Mater. Res. Bull., 2013, 48, 4032–4038 CrossRef CAS
.
- H. Okuzaki, H. Suzuki and T. Ito, J. Phys. Chem. B, 2009, 113, 11378–11383 CrossRef CAS PubMed
.
- F. Louwet, L. Groenendaal, J. Dhaen, J. Manca, J. van Luppen, E. Verdonck and L. Leenders, Synth. Met., 2003, 135–136, 115–117 CrossRef CAS
.
- L. Groenendaal, F. Jonas, D. Freitag, H. Pielartzik and J. R. Reynolds, Adv. Mater., 2000, 12, 481–494 CrossRef CAS
.
- A. Charba, M. Mumtaz, C. Brochon, H. Cramail, G. Hadziioannou and E. Cloutet, Langmuir, 2014, 30, 12474–12482 CrossRef CAS PubMed
.
- S. Roy, J. M. Fortier, R. Nagarajan, S. Tripathy, J. Kumar, L. A. Samuelson and F. F. Bruno, Biomacromolecules, 2002, 3, 937–941 CrossRef CAS PubMed
.
- D. G. Harman, R. Gorkin, L. Stevens, B. Thompson, K. Wagner, B. Weng, J. H. Y. Chung, M. In Het Panhuis and G. G. Wallace, Acta Biomater., 2015, 14, 33–42 CrossRef CAS PubMed
.
- S. Ghosh and O. Inganäs, Electrochem. Solid-State Lett., 2000, 3, 213–215 CrossRef CAS
.
- S. Ghosh and O. Inganäs, J. Electrochem. Soc., 2000, 147, 1872–1877 CrossRef CAS
.
- S. Ghosh and O. Inganäs, Adv. Mater., 1999, 11, 1214–1218 CrossRef CAS
.
- C. Yang and P. Liu, Ind. Eng. Chem. Res., 2009, 48, 9498–9503 CrossRef CAS
.
- J. Heinze, B. A. Frontana-Uribe and S. Ludwigs, Chem. Rev., 2010, 110, 4724–4771 CrossRef CAS PubMed
.
- S. Kirchmeyer and K. Reuter, J. Mater. Chem., 2005, 15, 2077–2088 RSC
.
- R. A. Lee, C. Bédard, V. Berberi, R. Beauchet and J.-M. Lavoie, Bioresour. Technol., 2013, 144, 658–663 CrossRef CAS PubMed
.
- Z. Li and Y. Ge, J. Braz. Chem. Soc., 2011, 22, 1866–1871 CrossRef CAS
.
- Lignin and Lignans: Advances in Chemistry.
- B. Park, L. Yang, E. M. J. Johansson, N. Vlachopoulos, A. Chams, C. Perruchot, M. Jouini, G. Boschloo and A. Hagfeldt, J. Phys. Chem. C, 2013, 117, 22484–22491 CAS
.
- J. J. Apperloo, L. “Bert” Groenendaal, H. Verheyen, M. Jayakannan, R. A. J. Janssen, A. Dkhissi, D. Beljonne, R. Lazzaroni and J.-L. Brédas, Chem.–Eur. J., 2002, 8, 2384–2396 CrossRef CAS
.
- R. B. Lima, R. Raza, H. Qin, J. Li, M. E. Lindström and B. Zhu, RSC Adv., 2013, 3, 5083–5089 RSC
.
- F. N. Ajjan, M. J. Jafari, T. Rębiś, T. Ederth and O. Inganäs, J. Mater. Chem. A, 2015, 3, 12927–12937 CAS
.
- C. Kvarnström, H. Neugebauer, A. Ivaska and N. S. Sariciftci, J. Mol. Struct., 2000, 521, 271–277 CrossRef
.
- L. Zhan, Z. Song, J. Zhang, J. Tang, H. Zhan, Y. Zhou and C. Zhan, Electrochim. Acta, 2008, 53, 8319–8323 CrossRef CAS
.
- P. Novák, K. Müller, K. S. V. Santhanam and O. Haas, Chem. Rev., 1997, 97, 207–282 CrossRef
.
- H. Olsson, E. Jämstorp Berg, M. Strømme and M. Sjödin, Electrochem. Commun., 2015, 50, 43–46 CrossRef CAS
.
- M. Quan, D. Sanchez, M. F. Wasylkiw and D. K. Smith, J. Am. Chem. Soc., 2007, 129, 12847–12856 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Lignin quantification, additional electrochemical characterization and SEM. See DOI: 10.1039/c5ta10096h |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |