 Open Access Article
Open Access ArticleA self-assembled intercalated metal–organic framework electrode with outstanding area capacity for high volumetric energy asymmetric capacitors†
Nobuhiro
Ogihara
*,
Yuka
Ozawa
and
Osamu
Hiruta
Toyota Central R&D Laboratories, Inc., Nagakute, Aichi 480-1192, Japan. E-mail: ogihara@mosk.tytlabs.co.jp
First published on 26th January 2016
Abstract
The enhancement of the energy of electrochemical capacitors while maintaining high power, long-term cycle stability and safety is challenging. Although there are several types of capacitors that realize high energy density, a system that achieves high energy with safety is still desired. Here we introduce a novel asymmetric capacitor with a negative electrode consisting of an intercalated metal–organic framework (iMOF) composed of 2,6-naphthalene dicarboxylate dilithium, which exhibits a flat plateau near 0.8 V vs. Li/Li+, suitable for high voltage as well as safety. Furthermore, for high volumetric energy density, we propose an extremely thick iMOF electrode prepared by self-assembly of the active material and conductive nanocarbon with an amphiphilic polymer, which possess efficient electron and Li+ transport pathways, and therefore exhibited an outstanding area capacity of over 2.5 mA h cm−2. Asymmetric capacitors with iMOF negative and activated carbon positive electrodes exhibited a high volumetric energy of 60 W h L−1 with favorable power and cycle stability.
Introduction
In energy storage technology for large-scale applications, electrochemical capacitors known as supercapacitors with sufficiently high power capability, which corresponds to a rapid charge–discharge response, play an important role in recovering much of the available energy that is otherwise released as heat.1,2 Electric double-layer capacitors (EDLCs) with identical electrodes composed of activated carbon (AC) with high specific surface area have such high power capability because their non-faradaic reactions associated with ion adsorption and desorption on the electrode surface exhibit excellent responses. However, EDLCs exhibit lower voltage (2.5 V) and lower energy density (ca. 10 W h L−1) than lithium (Li)-ion batteries. Pseudocapacitors containing electrochemical capacitors with high energy density have been proposed,3 such as manganese oxide (MnO2), ruthenium oxide (RuO2),4,5 nickel hydroxide (Ni(OH)2),6 two-dimensional transition metal carbides and nitrides (MXenes) in aqueous7–11 and non-aqueous12–14 electrolyte systems, molybdenum disulphide (MoS2) nanosheets in aqueous15 and non-aqueous16 electrolyte systems, and metal–organic frameworks (MOFs).17 However, these systems are currently limited by cell voltages of less than 1.2 V because of the aqueous electrolyte, and the need for thick electrodes increases their mass loading weight,8,16 so further research is needed.Asymmetric capacitors consisting of a faradaic intercalation electrode and a non-faradaic electrode in non-aqueous electrolytes have been proposed as an alternative to pseudocapacitors and EDLCs.18–20 Graphite,21 Li4Ti5O12,22–24 TiO2–B25 and T-Nb2O5 (ref. 20) have been used as intercalation negative electrodes for asymmetric capacitors. An asymmetric capacitor with a graphite electrode exhibited higher voltage close to 4 V and higher energy density (ca. 60 W h L−1) than EDLCs.21 However, graphite operating at 0.05 V (vs. Li/Li+) (Fig. 1) has a safety risk because Li-dendrite deposition can result in an internal short circuit during Li intercalation at a high charging rate18,26 and/or under low temperature conditions.27 As a result, the capacity used is limited to less than 20%.28 Meanwhile, Li4Ti5O12 operating at 1.55 V has an issue arising from the lower voltage design (1.4–2.8 V) and lower energy density (ca. 40 W h L−1) of the cell than graphite.22,23 Therefore, a negative electrode operating at an intermediate potential between those of graphite and Li4Ti5O12 would be desirable.
In this work, we report a novel type of asymmetric capacitor with intercalated MOF (iMOF) negative and AC positive electrodes (Fig. 1) to achieve high energy density and suppress the risk of Li-dendrite formation simultaneously, and a proposal of a high-mass loading weight electrode preparation process for the iMOF. Recently, we synthesized an iMOF of 2,6-naphthalene dicarboxylate dilithium crystal (2,6-Naph(COOLi)2) under mild conditions (see the Sample synthesis in Methods).29–31 Unlike other reported pseudocapacitive MOF electrode materials, 2,6-Naph(COOLi)2 is nonporous in nature32 and shows a lithium intercalation reaction with two electron transfer of π-conjugated dicarboxylate operating at 0.8 V, which is between the potentials of graphite and Li4Ti5O12 (Fig. 1). As shown in Fig. 2, the organic–inorganic layered framework of 2,6-Naph(COOLi)2 was composed of π-stacked naphthalene groups and a tetrahedral LiO4 network of the carboxylate salt, respectively, providing chemical and electrochemical stability. 2,6-Naph(COOLi)2 has a high density of ca. 1.6 g cm−3, reversible specific capacity of 220 mA h g−1 and remarkably small volume change (0.33%) during Li intercalation, which is one order of magnitude lower than that of other intercalation electrode materials. By our detailed structural analysis of the lithium intercalated state,29 the proposed material possesses the tetrahedral LiO3C network and Li-doped naphthalene packing, which are primarily responsible for Li transport and electronic conduction pathways. Thereby the framework of 2,6-Naph(COOLi)2 (Fig. 2) provides good cycle stability and 2D pathways for both electron and Li+ transport, allowing a facile redox response.
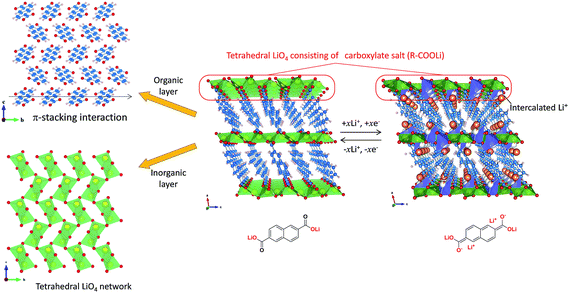 | ||
| Fig. 2 Structure of the before and after lithium-intercalated 2,6-Naph(COOLi)2 taken from their XRD patterns.29 Intercalated Li (orange), Li (yellow), O (red), C (blue), and H (white). | ||
To design asymmetric capacitors with high energy density, the area capacity ratio of positive and negative electrodes should be optimized at a high loading weight of the active material according to eqn (1).
| Cap.negativemnegative/Cap.positivempositive | (1) |
Experimental
Sample synthesis
The synthesis of 2,6-naphthalene dicarboxylate dilithium (2,6-Naph(COOLi)2) was reported previously.29 2,6-Naph(COOLi)2 was synthesized from lithium hydroxide monohydrate (4.85 g, 115.6 mmol) and 2,6-naphthalenedicarboxylic acid (10.0 g, 46.3 mmol) heated under reflux in 300 mL methanol. The solution was initially clear, and then a white precipitate formed over the course of 30 min. The suspension was stirred under reflux conditions for 12 h. The mixture was evaporated, and then the resulting solid was washed with methanol, and dried under ambient conditions. The product was obtained as needle-shaped white crystals (96.8% yield based on 2,6-Naph(COOLi)2).Electrode and electrolyte preparations
Amphiphilic polymer-based 2,6-Naph(COOLi)2 electrodes were prepared by coating a dispersion of 77–82 wt% 2,6-Naph(COOLi)2, 13–14 wt% carbon black as a conductive agent, 2–6 wt% carboxymethylcellulose (CMC) or polyacrylic acid (PAA) as an amphiphilic polymer, and 2–4 wt% styrene butadiene rubber (SBR) as a binder in water onto Cu foil. Electrode loading weights of ca. 2, 4, 6, 8, 12 and 14 mg cm−2 were used. Conventional PVDF-based 2,6-Naph(COOLi)2 electrodes were prepared by coating a dispersion of 79.6 wt% 2,6-Naph(COOLi)2, 13.0 wt% carbon black or carbon black with vapor-grown carbon fibers as a conductive agent, and 7.4 wt% PVDF binder in N-methyl-2-pyrrolidone onto Cu foil for comparison. The electrode loading weight was ca. 2 mg cm−2. Activated carbon (AC)-based electrodes were also prepared by coating a dispersion of 83 wt% AC (Kurare YP-20), 10.7 wt% carbon black, 4 wt% CMC, and 2.3 wt% SBR in water onto Al foil. The electrode loading weight was ca. 2 or 3 mg cm−2. All electrodes were dried at 120 °C under vacuum for at least 10 h before constructing electrochemical cells. The electrolytes in this study were 1 M LiPF6 dissolved in a solution of ethylene carbonate (EC), dimethyl carbonate (DMC), and ethyl methyl carbonate (EMC) (volume ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, respectively) and 1 M triethylmethylammonium tetrafluoroborate (NEt3MeBF4) dissolved in a solution of propylene carbonate. A microporous polypropylene film was used as the separator.
30, respectively) and 1 M triethylmethylammonium tetrafluoroborate (NEt3MeBF4) dissolved in a solution of propylene carbonate. A microporous polypropylene film was used as the separator.
Morphology characterization
The surface morphology of 2,6-Naph(COOLi)2 crystals and 2,6-Naph(COOLi)2 electrodes was examined by scanning electron microscopy (SEM, S-5500, Hitachi, Japan). Samples for cross-sectional SEM analysis were prepared by an in situ focused ion beam (FIB) technique. Images were recorded using a SEM (NB5000 nanoDUE'T FIB-SEM, Hitachi) operating at 2 kW. Cross-sectional images and elemental mapping of the 2,6-Naph(COOLi)2 electrodes were obtained by electron probe microanalysis (EPMA) (JXA-8500F JEOL, Japan) using an acceleration voltage of 7 kV and beam current of 50 nA. Prior to EPMA, the samples were embedded in epoxy resin (EpoFix, Struers) and cross-sectional samples were prepared using a FIB.Electrochemical characterization
The electrochemical properties of Li/2,6-Naph(COOLi)2 and 2,6-Naph(COOLi)2/AC cells were examined using coin- and laminate-type cells, respectively, assembled with a separator filled with an LiPF6-based electrolyte in an argon-filled glove box. In galvanostatic charge–discharge measurements to determine the specific capacity and electrochemical reversibility of the 2,6-Naph(COOLi)2 electrodes at 25 °C, coin-type cells were cycled between 0.5 and 2.0 V vs. Li/Li+ at a rate corresponding to fully charging the theoretical capacity of 2,6-Naph(COOLi)2 per 10 h at ca. 2, 4, 6 and 8 mg cm−2 and 20 h at ca. 12 and 14 mg cm−2. For laminate-type cells, an AC electrode was used as the positive electrode, and a Li pre-doped 2,6-Naph(COOLi)2 electrode was used as the negative electrode. The electrolyte was the same as that in the coin-type cells. For comparison, EDLCs with identical AC electrodes were assembled with a separator filled with a NEt3MeBF4-based electrolyte. Galvanostatic charge–discharge measurements of the 2,6-Naph(COOLi)2/AC asymmetric capacitors and EDLCs were conducted at 25 °C between 1.5 and 3.8 V, and 0.0 and 2.5 V, respectively, at various current densities between 1 and 100 mA cm−2 to determine energy and power densities. Prior to galvanostatic charge–discharge measurements of the asymmetric capacitors, cells containing 2,6-Naph(COOLi)2 and Li metal electrodes with a separator were prepared. These cells were cycled between 0.5 and 2.0 V under the same rate conditions as the coin-type cells and the state of charge (SOC) of the 2,6-Naph(COOLi)2 electrodes was set at 50%. Asymmetric capacitors were then prepared using the 2,6-Naph(COOLi)2 electrodes taken from these cells and the AC electrodes.Electrochemical impedance spectroscopy using a symmetric cell35,41 was performed to determine the ionic resistance (Rion) of the porous 2,6-Naph(COOLi)2 electrodes. To do this, identical 2,6-Naph(COOLi)2 electrodes were assembled with a separator filled with a LiPF6-based electrolyte. Electrochemical impedance measurements (Solatron 1260/1286, England) of symmetric cells were performed at an open-circuit potential. The frequency was varied from 100 kHz to 100 mHz with a perturbation amplitude of 10 mV (peak to peak). Measurements were performed between −10 and 60 °C.
Results and discussion
Firstly, we prepare extremely thick electrodes having a high electrochemical activity through self-assembly using amphiphilic polymers, iMOF particles and conductive nanocarbon materials. The schematic illustration in Fig. 3a shows the self-assembled electrode preparation procedure. The crystal surface of 2,6-Naph(COOLi)2 shows hydrophilic properties because of carboxylate units (Fig. 2). In contrast, the surface of the conducting nanocarbon shows hydrophobic properties. Therefore, the self-assembly between 2,6-Naph(COOLi)2 and conductive nanocarbon was attempted by hydrophilic and hydrophobic interactions of amphiphilic polymers in aqueous slurry for electrode coating. As shown in Fig. 3b, aqueous slurries of 2,6-Naph(COOLi)2 and conductive nanocarbon with and without the amphiphilic polymer are black and gray, respectively. This indicates that the white 2,6-Naph(COOLi)2 particles are covered by black conductive nanocarbon in the slurry with the amphiphilic polymer, whereas the surface of 2,6-Naph(COOLi)2 is exposed in the slurry without the amphiphilic polymer. This means that the hydrophilic carboxylate groups on the side chains and the hydrophobic main chain of the amphiphilic polymer interact with carboxylate groups on the surface of the 2,6-Naph(COOLi)2 crystal and hydrophobic conductive nanocarbon in solution, respectively. At low carbon contents, cross-sectional images of the electrodes prepared by coating of the self-assembled aqueous slurry confirmed that 2,6-Naph(COOLi)2 particles were covered with conductive nanocarbon and revealed uniform pore structures in the depth direction (Fig. 3c and d). Although pristine 2,6-Naph(COOLi)2 consists of needle-shaped crystals with hundreds of nanosized irregularities resembling intestinal villus (Fig. S1†), the irregularities in the self-assembled electrode are coated with conductive nanocarbon (Fig. S2†). This indicates that an optimal electron transfer interface can form between 2,6-Naph(COOLi)2 and conductive nanocarbon through self-assembly driven by an amphiphilic polymer. Conversely, aggregation of the conductive nanocarbon was observed without covering 2,6-Naph(COOLi)2 particles in the electrode prepared using the conventional polyvinylidene fluoride (PVDF) binder (Fig. S3†).Next, the electrochemical performance of the self-assembled 2,6-Naph(COOLi)2 electrode was examined in cells using Li metal as the counter electrode. The self-assembled 2,6-Naph(COOLi)2 electrode has advantages of (i) low conductive nanocarbon content, (ii) high mass-loading weight and (iii) reversible electrochemistry. As shown in Fig. S4,† charge–discharge profiles measured for Li/2,6-Naph(COOLi)2 cells confirmed that the 2,6-Naph(COOLi)2 electrode prepared using the amphiphilic polymer CMC possessed higher specific capacity, which corresponds to approximately the theoretical capacity of 2e− and 2Li+ per molecule, compared to that prepared using PVDF under the same conditions, and favorable rate capabilities (Fig. S5†). This means that the self-assembled 2,6-Naph(COOLi)2 electrode gives a reversible redox response at a small amount of conductive carbon ratio by uniform coating of conductive carbon onto 2,6-Naph(COOLi)2 particles, as shown in Fig. 3c and d.
Importantly, as shown in Fig. 4a, the self-assembled 2,6-Naph(COOLi)2 electrode maintained high reversible specific capacities and small polarization at a high loading weight of ca. 14 mg cm−2. The area capacity increased linearly with loading weight, and reached an outstanding value of 2.66 mA h cm−2 (Fig. 4b). This value is five times higher than those reported for organic electrode materials of Li-based (0.5–0.7 mA h cm−2)42–45 and Na-based (0.2–0.5 mA h cm−2)46–49 electrolyte systems (Fig. 5), and comparable to those of high-capacity silicon-based negative electrodes for Li-ion batteries (0.13–5.00 mA h cm−2) (Fig. S6†).
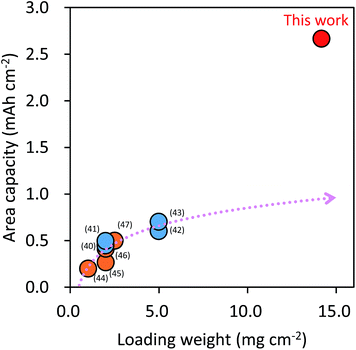 | ||
| Fig. 5 Comparison of the area capacity of 2,6-Naph(COOLi)2 electrodes with a high loading weight with those of other reported organic redox electrode materials (blue: Li-base,42–45 and orange: Na-base46–49). The arrow is an approximate curve estimated by the least squares method in the reported values. | ||
To gain further insight into the ionic resistance in pores (Rion) and the corresponding ionic conductivity of the prepared porous 2,6-Naph(COOLi)2 electrodes, electrochemical impedance spectroscopy using symmetric cells that assemble the identical 2,6-Naph(COOLi)2 electrodes with the separator filled with the electrolyte35,41 was conducted. This technique can focus on the impedance of a single electrode without affecting the other electrodes. Although the electrodes with high loading weights of ca. 12 and 14 mg cm−2 exhibited relatively high resistance at high frequencies of over 1 kHz (Fig. S7†), as shown in Fig. 6a, the plots were linear with a 45° slope and vertical from the real axis in the middle- and low-frequency regions, respectively, which is the typical electrical blocking behavior of porous electrodes without a charge–transfer reaction. Rion obtained by fitting (see the ESI and Fig. S8†) increased linearly with loading weight (Fig. 6b). This indicates an ideal ionic resistance related to the mass-loading weight as shown in eqn (S3) in the ESI.† Therefore, the ionic conductivity in electrode pores was constant for all electrode thicknesses (Fig. 6b) and also exhibited identical temperature dependence regardless of loading weight (Fig. S9†). The use of CMC and its analogs as binders has demonstrated improvement of cycle stability due to high adhesion for graphite carbon or silicon negative electrode materials.50–53 Unlike the previous reports, key finding of this study is that amphiphilic polymers have a high selectivity for both the iMOF and conducting carbon surfaces, whereas hydrophobic PVDF selects only the hydrophobic conducting carbon. Thereby, the conductive carbon is uniformly coated on the iMOF surface in small amounts of conducting carbons with amphiphilic polymers. Therefore, the self-assembled iMOF electrode can lead to practically useful electrochemical performance in a wide range of loading weights.
Finally, an asymmetric capacitor composed of Li-pre-doped 2,6-Naph(COOLi)2 negative and AC positive electrodes was constructed in a laminate-type cell. As shown in Fig. 7a, the asymmetric capacitor exhibited a higher voltage range of 1.5–3.8 V compared with the EDLC. This asymmetric capacitor utilized the high electric double-layer capacitances of both PF6− anions and Li+ cations at the AC positive electrode by the pre-doping treatment with Li. This has been confirmed by the results of the differential capacity dQ/dV plots of the same cells (Fig. S10†). The asymmetric capacitor also showed long-term cycle stability with a retention of 86% after 1000 cycles (Fig. 7b). Previously we have confirmed that there is no change of obvious size, shape and crystal structure before and after cycling.29 This result in this study suggests that the favorable cycle performances of the proposed asymmetric capacitor are attributed to a very small volume strain during Li intercalation and the insolubility of the self-assembled 2,6-Naph(COOLi)2 electrode in common organic electrolytes. The energy and power performances of the capacitor were optimized according to eqn (1) for preferable electrode conditions36 (Fig. S11†). The capacitor can be discharged under high rate conditions (discharge for 1 min at 60C) (Fig. 7c, inset). The retention rate at 60C is ca. 60% (Fig. 7c), which is close to that of the pseudocapacitive electrode material orthorhombic T-Nb2O5, which has high rate capability,20 and better than that of other Na-based asymmetric capacitors with MXene electrode materials, such as a Ti2CTx (negative)/Na2Fe(S4O4)3 (positive) capacitor54 or a hard carbon/V2C capacitor.14 The proposed asymmetric capacitor exhibited high gravimetric and volumetric energy (ca. 80 W h kg−1 and 60 W h L−1, respectively) and gravimetric and volumetric power (ca. 3.2 kW kg−1 and 2.3 kW L−1, respectively) (Fig. 7d). Overall, the capacitor exhibits an energy density that is five to six times higher than that of typical EDLCs as well as comparable power density, higher power density than a graphite/AC capacitor (ca. 1.2 kW L−1),21 and higher energy density than a Li4Ti5O12/AC capacitor (ca. 55 W h kg−1, 40 W h L−1).22 Although these results reveal excellent full-cell performance compared to reported asymmetric capacitors optimized by Li pre-doping21 or using nanosized active materials22 to maximize rate capability, the power capability of the proposed asymmetric capacitors can still be further improved.
 | ||
Fig. 7 (a) Galvanostatic charge–discharge curves of the proposed asymmetric capacitor (iMOF-capacitor) and EDLC at the same loading weight for an AC positive electrode and current density. (b) Cycling capacity retention of asymmetric capacitors at a charge–discharge rate of 10C. The capacity ratio of the negative electrode to the positive electrode was 2.7. The ratio of active material to conductive nanocarbon was (blue) 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, (green) 81 15, (green) 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19, (yellow) 77 19, (yellow) 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 and (red) 74 23 and (red) 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26. (c) Rate capability of the optimized cell is shown in Fig. S11.† Inset: discharge curves of the same cell at rates from 1 to 60C. (d) Comparison of volumetric energy versus power of the asymmetric capacitor, EDLC and other reported asymmetric capacitors, such as graphite/AC21 and Li4Ti5O12/AC22 cells. 26. (c) Rate capability of the optimized cell is shown in Fig. S11.† Inset: discharge curves of the same cell at rates from 1 to 60C. (d) Comparison of volumetric energy versus power of the asymmetric capacitor, EDLC and other reported asymmetric capacitors, such as graphite/AC21 and Li4Ti5O12/AC22 cells. | ||
Conclusions
In summary, the use of an iMOF, 2,6-Naph(COOLi)2, electrode material was demonstrated as an effective approach for advanced asymmetric capacitors. This work developed an outstanding area capacity on the iMOF electrode through self-assembly using amphiphilic polymers, which allows efficient electron and Li+ transport not only in the bulk crystal but also in the porous electrodes. Therefore, the proposed asymmetric capacitors with iMOF electrodes possess both high volumetric energy and power with safety. This study is a new approach to organic-based electrodes. Thus, we believe that our finding of self-assembled thick electrodes composed of iMOF not only reveals the feasibility of organic-based electrode materials, but also advances the evolution of supercapacitors.Acknowledgements
We acknowledge Ms. Y. Matsuoka, Mr J. Seki and Mr Y. Yagi for morphology characterization and Dr T. Sasaki for fruitful discussion.References
- J. R. Miller and P. Simon, Science, 2008, 321, 651–652 CrossRef CAS PubMed.
- P. Simon, Y. Gogotsi and B. Dunn, Science, 2014, 343, 1210–1211 CrossRef CAS PubMed.
- V. Augustyn, P. Simon and B. Dunn, Energy Environ. Sci., 2014, 7, 1597 CAS.
- J. P. Zheng, P. J. Cygan and T. R. Jow, J. Electrochem. Soc., 1995, 142, 2699–2703 CrossRef CAS.
- W. Sugimoto, H. Iwata, Y. Yasunaga, Y. Murakami and Y. Takasu, Angew. Chem., Int. Ed. Engl., 2003, 42, 4092–4096 CrossRef CAS PubMed.
- H. B. Li, M. H. Yu, F. X. Wang, P. Liu, Y. Liang, J. Xiao, C. X. Wang, Y. X. Tong and G. W. Yang, Nat. Commun., 2013, 4, 1894 CrossRef CAS PubMed.
- M. R. Lukatskaya, O. Mashtalir, C. E. Ren, Y. Dall'Agnese, P. Rozier, P. L. Taberna, M. Naguib, P. Simon, M. W. Barsoum and Y. Gogotsi, Science, 2013, 341, 1502–1505 CrossRef CAS PubMed.
- M. Ghidiu, M. R. Lukatskaya, M. Q. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2014, 516, 78–81 CAS.
- Z. Ling, C. E. Ren, M.-Q. Zhao, J. Yang, J. M. Giammarco, J. Qiu, M. W. Barsoum and Y. Gogotsi, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 16676–16681 CrossRef CAS PubMed.
- M. Q. Zhao, C. E. Ren, Z. Ling, M. R. Lukatskaya, C. Zhang, K. L. Van Aken, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2015, 27, 339–345 CrossRef CAS PubMed.
- M. R. Lukatskaya, S.-M. Bak, X. Yu, X.-Q. Yang, M. W. Barsoum and Y. Gogotsi, Adv. Energy Mater., 2015, 5, 1500589 Search PubMed.
- M. Naguib, J. Come, B. Dyatkin, V. Presser, P.-L. Taberna, P. Simon, M. W. Barsoum and Y. Gogotsi, Electrochem. Commun., 2012, 16, 61–64 CrossRef CAS.
- M. Naguib, J. Halim, J. Lu, K. M. Cook, L. Hultman, Y. Gogotsi and M. W. Barsoum, J. Am. Chem. Soc., 2013, 135, 15966–15969 CrossRef CAS PubMed.
- Y. Dall'Agnese, P. L. Taberna, Y. Gogotsi and P. Simon, J. Phys. Chem. Lett., 2015, 6, 2305–2309 CrossRef PubMed.
- H. Tang, J. Wang, H. Yin, H. Zhao, D. Wang and Z. Tang, Adv. Mater., 2015, 27, 1117–1123 CrossRef CAS PubMed.
- M. Acerce, D. Voiry and M. Chhowalla, Nat. Nanotechnol., 2015, 10, 313–318 CrossRef CAS PubMed.
- K. M. Choi, H. M. Jeong, J. H. Park, Y.-B. Zhang, J. K. Kang and O. M. Yaghi, ACS Nano, 2014, 8, 7451–7457 CrossRef CAS PubMed.
- I. Plitz, A. DuPasquier, F. Badway, J. Gural, N. Pereira, A. Gmitter and G. G. Amatucci, Appl. Phys. A, 2005, 82, 615–626 CrossRef.
- A. Yoshino, T. Tsubata, M. Shimoyamada, H. Satake, Y. Okano, S. Mori and S. Yata, J. Electrochem. Soc., 2004, 151, A2180 CrossRef CAS.
- V. Augustyn, J. Come, M. A. Lowe, J. W. Kim, P. L. Taberna, S. H. Tolbert, H. D. Abruna, P. Simon and B. Dunn, Nat. Mater., 2013, 12, 518–522 CrossRef CAS PubMed.
- J. Zhang, Z. Q. Shi and C. Y. Wang, Electrochim. Acta, 2014, 125, 22–28 CrossRef CAS.
- K. Naoi, S. Ishimoto, J.-I. Miyamoto and W. Naoi, Energy Environ. Sci., 2012, 5, 9363 CAS.
- K. Naoi, S. Ishimoto, Y. Isobe and S. Aoyagi, J. Power Sources, 2010, 195, 6250–6254 CrossRef CAS.
- H.-G. Jung, N. Venugopal, B. Scrosati and Y.-K. Sun, J. Power Sources, 2013, 221, 266–271 CrossRef CAS.
- V. Aravindan, N. Shubha, W. C. Ling and S. Madhavi, J. Mater. Chem. A, 2013, 1, 6145–6151 CAS.
- L. Yang, X. Cheng, Y. Gao, P. Zuo, Y. Ma, C. Du, B. Shen, Y. Cui, T. Guan and G. Yin, ACS Appl. Mater. Interfaces, 2014, 6, 12962–12970 CAS.
- G. Park, N. Gunawardhana, H. Nakamura, Y.-S. Lee and M. Yoshio, J. Power Sources, 2012, 199, 293–299 CrossRef CAS.
- V. Khomenko, E. Raymundo-Piñero and F. Béguin, J. Power Sources, 2008, 177, 643–651 CrossRef CAS.
- N. Ogihara, T. Yasuda, Y. Kishida, T. Ohsuna, K. Miyamoto and N. Ohba, Angew. Chem., Int. Ed. Engl., 2014, 53, 11467–11472 CrossRef CAS PubMed.
- T. Yasuda and N. Ogihara, Chem. Commun., 2014, 50, 11565–11567 RSC.
- N. Ogihara and Y. Kishida, Electrochemistry, 2015, 83, 861–863 CrossRef CAS.
- D. Banerjee, S. J. Kim and J. B. Parise, Cryst. Growth Des., 2009, 9, 2500–2503 CAS.
- Z. Zhang, H. Yoshikawa and K. Awaga, J. Am. Chem. Soc., 2014, 136, 16112–16115 CrossRef CAS PubMed.
- S. Gottis, A. L. Barres, F. Dolhem and P. Poizot, ACS Appl. Mater. Interfaces, 2014, 6, 10870–10876 CAS.
- N. Ogihara, Y. Itou, T. Sasaki and Y. Takeuchi, J. Phys. Chem. C, 2015, 119, 4612–4619 CAS.
- Y. Gogotsi and P. Simon, Science, 2011, 334, 917–918 CrossRef CAS PubMed.
- C. Wang, Z. Wang and X. Zhang, Acc. Chem. Res., 2012, 45, 608–618 CrossRef CAS PubMed.
- W.-C. Huang, S.-Y. Chen and D.-M. Liu, Soft Matter, 2012, 8, 10868 RSC.
- W. C. Huang, H. Y. Lai, L. W. Kuo, C. H. Liao, P. H. Chang, T. C. Liu, S. Y. Chen and Y. Y. Chen, Adv. Mater., 2015, 27, 4186–4193 CrossRef CAS PubMed.
- X. Wang, Y. Guo, D. Li, H. Chen and R. C. Sun, Chem. Commun., 2012, 48, 5569–5571 RSC.
- N. Ogihara, S. Kawauchi, C. Okuda, Y. Itou, Y. Takeuchi and Y. Ukyo, J. Electrochem. Soc., 2012, 159, A1034–A1039 CrossRef CAS.
- S. Wang, L. Wang, K. Zhang, Z. Zhu, Z. Tao and J. Chen, Nano Lett., 2013, 13, 4404–4409 CrossRef CAS PubMed.
- Z. Song, Y. Qian, X. Liu, T. Zhang, Y. Zhu, H. Yu, M. Otani and H. Zhou, Energy Environ. Sci., 2014, 7, 4077–4086 CAS.
- W. Walker, S. Grugeon, O. Mentre, S. Laruelle, J.-M. Tarascon and F. Wudl, J. Am. Chem. Soc., 2010, 132, 6517–6523 CrossRef CAS PubMed.
- W. Walker, S. Grugeon, H. Vezin, S. Laruelle, M. Armand, F. Wudl and J.-M. Tarascon, J. Mater. Chem., 2011, 21, 1615 RSC.
- A. Choi, Y. K. Kim, T. K. Kim, M. S. Kwon, K. T. Lee and H. R. Moon, J. Mater. Chem. A, 2014, 2, 14986–14993 CAS.
- W. Luo, M. Allen, V. Raju and X. L. Ji, Adv. Energy Mater., 2014, 4, 1400554 Search PubMed.
- S. Wang, L. Wang, Z. Zhu, Z. Hu, Q. Zhao and J. Chen, Angew. Chem., Int. Ed. Engl., 2014, 53, 5892–5896 CrossRef CAS PubMed.
- A. Abouimrane, W. Weng, H. Eltayeb, Y. Cui, J. Niklas, O. Poluektov and K. Amine, Energy Environ. Sci., 2012, 5, 9632–9638 CAS.
- H. Buqa, M. Holzapfel, F. Krumeich, C. Veit and P. Novák, J. Power Sources, 2006, 161, 617–622 CrossRef CAS.
- J. Li, R. B. Lewis and J. R. Dahn, Electrochem. Solid-State Lett., 2007, 10, A17–A20 CrossRef CAS.
- I. Kovalenko, B. Zdyrko, A. Magasinski, B. Hertzberg, Z. Milicev, R. Burtovyy, I. Luzinov and G. Yushin, Science, 2011, 334, 75–79 CrossRef CAS PubMed.
- L. Jabbour, C. Gerbaldi, D. Chaussy, E. Zeno, S. Bodoardo and D. Beneventi, J. Mater. Chem., 2010, 20, 7344 RSC.
- X. Wang, S. Kajiyama, H. Iinuma, E. Hosono, S. Oro, I. Moriguchi, M. Okubo and A. Yamada, Nat. Commun., 2015, 6, 6544 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ta09559j |
| This journal is © The Royal Society of Chemistry 2016 |




