 Open Access Article
Open Access ArticleCO2 conversion: the potential of porous-organic polymers (POPs) for catalytic CO2–epoxide insertion†
Mohamed H.
Alkordi‡§
a,
Łukasz J.
Weseliński‡¶
a,
Valerio
D'Elia||
b,
Samir
Barman
b,
Amandine
Cadiau
a,
Mohamed N.
Hedhili
c,
Amy J.
Cairns
a,
Rasha G.
AbdulHalim
a,
Jean-Marie
Basset
b and
Mohamed
Eddaoudi
*a
aFunctional Materials Design, Discovery and Development Research Group (FMD3), Advanced Membranes and Porous Materials Center (AMPMC), Division of Physical Sciences and Engineering, King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Kingdom of Saudi Arabia. E-mail: mohamed.eddaoudi@kaust.edu.sa
bKAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Kingdom of Saudi Arabia
cImaging and Characterization Laboratory, King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Kingdom of Saudi Arabia
First published on 30th March 2016
Abstract
Novel porous organic polymers (POPs) have been synthesized using functionalized Cr and Co–salen complexes as molecular building blocks. The integration of metalosalen catalysts into the porous polymer backbone permits the successful utilization of the resultant functionalized material as a solid-state catalyst for CO2–epoxide cycloaddition reactions with excellent catalytic performance under mild conditions of temperature and pressure. The catalysts proved to be fully recyclable and robust, thus showing the potential of POPs as smart functional materials for the heterogenization of key catalytic elements.
1. Introduction
CO2 reutilization is regarded as a plausible strategy to mitigate the waste CO2 emissions from energy intensive industrial and human activities.1 Therefore, the deployment of advanced technologies aiming to exploit CO2 as a feedstock for the synthesis of valuable chemical products is highly desirable.2 In this context, the synthesis of industrially relevant3 cyclic organic carbonates from CO2 and epoxides is gaining interest as a process that can be implemented under relatively mild conditions using recently developed homogeneous catalysts.4,5 Nevertheless, the immobilization of such catalytic moieties to yield recoverable and reusable catalysts with preserved activity and selectivity remains an ongoing challenge.Towards this goal, novel porous materials with adequate pore volume and surface area, strong affinity to CO2, and appropriate stability are being sought as prospective candidates to tackle this challenge. Porous-Organic Polymers (POPs) represent a promising class of sorbents that can fulfill the abovementioned criteria.6 Interestingly, the ability to assemble POPs using the molecular building block (MBB) approach offers potential to fine-tune their chemical composition and the resultant pore size, shape and functionality to address a given targeted application. Markedly, the predominant covalent character of these materials offers high thermal and chemical stability that permits the potential utilization of POPs under practical reaction conditions. Rationally, the potential POPs were recognized for applications pertaining to gas storage and separation,6g,7 photoelectronics8 and catalysis.9
Salen complexes are widely recognized in catalysis10 and proven to be effective homogeneous catalysts for the cycloaddition of CO2 to epoxides.11 Interest in heterogeneous metalosalen-based catalysts is largely driven by the quest to alleviate two major limitations commonly encountered in homogeneous catalysis, namely difficult separation/recovery of the catalyst from the reaction mixture and the limited catalyst lifetime due to either bimolecular deactivation leading to bridged μ-oxide dimers10b (hindered access to catalytic sites) and/or oxidative self-degradation. Therefore, the immobilization of metalosalens on solid matrices provides a promising approach to isolate and protect the catalytic sites of the metallacycle, and subsequently overcome the aforementioned drawbacks.
A few examples on the application of metalosalen-POPs as heterogeneous catalysts for the title cycloaddition have been recently reported.12 However, only few compounds have been investigated in spite of a large variety of metalosalens conceivable, depending on the choice of the metal, organic backbone and counter-ion. Here, we report the application of novel metalosalen-containing POPs as heterogeneous catalysts for the synthesis of cyclic carbonates from epoxides and CO2. The reported catalysts showed remarkable catalytic activities, under relatively mild reaction conditions, and excellent recyclability properties, i.e. convenient separation and multiple reuse of the material.
2. Experimental
Materials and methods
All reagents were commercially available and used as received unless further noted. NMR spectra were recorded at room temperature with a Bruker Avance III 500 MHz spectrometer using CDCl3 as a solvent. Cross polarization magic angle spinning (CP-MAS) solid state 13C NMR spectra were recorded with a Bruker Avance 600 WB spectrometer. Elemental analysis was performed with a Thermo Scientific Flash 2000 instrument. Powder X-ray diffraction (PXRD) measurements were carried out at room temperature on a PANalytical X'Pert PRO diffractometer 45 kV, 40 mA for CuKα (λ = 1.5418 Å) with a scan speed of 0.02° min−1 and a step size of 0.008° in 2θ. UV spectra were recorded with a Perkin Elmer Lambda 950 UV/VIS spectrometer. Thermogravimetric analysis (TGA) measurements were performed on a TA Q500 apparatus, under air atmosphere (flow = 25 cm3 min−l, heating rate 5 °C min−1). Fourier-transform infrared (FT-IR) spectra (4000–600 cm−1) were recorded on a Thermo Scientific Nicolet 6700 apparatus. Low-pressure gas adsorption measurements were performed on a fully automated Autosorb 6B (for N2 sorption screening) and an Autosorb-iQ gas adsorption analyzer (Quantachrome Instruments) at pressures up to 1 atm. The cryogenic temperatures 77 K and 87 K were attained using liquid nitrogen and argon baths, respectively. Pore size distributions were calculated using a QSDFT pore size analysis method for carbons with slit-shaped/cylindrical/spherical pores. ICP-OES measurements of the metal loading on a porous material were carried out on a Varian 720-ES instrument after digestion of a 10 mg sample in aqua regia. XPS studies were carried out on a Kratos Axis Ultra DLD spectrometer equipped with a monochromatic Al Kα X-ray source (hν = 1486.6 eV) operating at 150 W, a multichannel plate and a delay line detector under a vacuum of 1–10−9 mbar. The survey and high-resolution spectra were collected at fixed analyzer pass energies of 160 eV and 20 eV, respectively. Samples were mounted in a floating mode in order to avoid differential charging. Charge neutralization was required for all samples. Binding energies were referenced to the aromatic sp2 hybridized (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) carbon for the C 1s peak set at 284.5 eV. The morphology of samples was determined by using a field-emission scanning electron microscope (FESEM, FEI Nova Nano 630). The chemical compositions of samples were analyzed by using an energy dispersive spectrometer (EDX, Quanta 600).
C) carbon for the C 1s peak set at 284.5 eV. The morphology of samples was determined by using a field-emission scanning electron microscope (FESEM, FEI Nova Nano 630). The chemical compositions of samples were analyzed by using an energy dispersive spectrometer (EDX, Quanta 600).
Synthesis of N,N′-bis(5-bromosalicylaldehyde)cyclohexane-diimine
The compound was prepared following a reported procedure.13 A suspension of trans-cyclohexane-1,2-diamine (1.71 g, 15 mmol) and 5-bromo-2-hydroxybenzaldehyde (6.03 g, 30 mmol) in absolute ethanol (20 ml) was vigorously stirred at 75 °C under Ar for 14 h. It was cooled, filtered on paper, washed thoroughly with ethanol and dried on air to produce a yellow solid, 6.56 g (97%). 1H NMR (500 MHz, CDCl3) δ = 13.3 (s, 2H), 8.18 (s, 2H), 7.33 (dd, J = 2.4, 8.8 Hz, 2H), 7.26 (s, 2H), 6.80 (d, J = 8.8 Hz, 2H), 3.33 (m, 2H), 1.90 (m, 4H), 1.74 (m, 2H), and 1.47 (m, J = 9.9 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ = 163.6, 160.1, 135.1, 133.7, 120.0, 119.0, 110.3, 72.8, 33.0, and 24.2.Synthesis of 1
The material was synthesized following the reported procedure.14 In a 150 ml oven-dried round bottom flask, a solution of N,N′-bis(5-bromosalicylaldehyde)cyclohexanediimine (0.96 g, 2 mmol) in dry DMF (30 ml, stored over CaH2) and dry triethylamine (6 ml) was bubbled with Ar for 30 min. Then, 1,3,5-triethynylbenzene (0.3 g, 2 mmol), PdCl2(PPh3)2 (44 mg, 0.063 mmol), and CuI (12 mg, 0.063 mmol) were added in one portion, the flask was evacuated/backfilled with Ar three times, sealed with septum and heated at 80 °C for 50 h with vigorous stirring. It was cooled, filtered, solid washed sequentially with DMF, water, THF, MeOH and acetone, then suspended in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 THF/CH2Cl2 (80 ml), and stirred at rt for 48 h, refreshing solution twice daily. It was filtered, washed with THF and dried at high vacuum at 60 °C overnight to give red-brown solid, 0.57 g. Calc. for C33H26N2O2: C, 82.13%; H, 5.43%; N, 5.81%; O, 6.63%. Found C, 79.8%; H, 4.58%; N, 4.16%. The proposed elemental composition is based on a 1
1 THF/CH2Cl2 (80 ml), and stirred at rt for 48 h, refreshing solution twice daily. It was filtered, washed with THF and dried at high vacuum at 60 °C overnight to give red-brown solid, 0.57 g. Calc. for C33H26N2O2: C, 82.13%; H, 5.43%; N, 5.81%; O, 6.63%. Found C, 79.8%; H, 4.58%; N, 4.16%. The proposed elemental composition is based on a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reaction between the salen moiety and the polytopic 1,3,5-triethynylbenzene linker. As suggested in other studies,14 factors such as chain termination by halogen atoms and residual halogen impurities, variable chain length, incomplete metalation and, possibly, homocoupling between the polytopic linker units can lead to a discrepancy between the elemental analysis values and those expected based on the stoichiometry of copolymerization. This is observed in this case and also for 1-Co and 1-Cr. In particular, this may result in a significant relative deviation with regards to the proposed N content as it is present in small proportions relative to C. Nevertheless, the ICP, EDX and XPS analyses (vide infra) confirmed the expected metal content and the expected N/metal ratio.
1 reaction between the salen moiety and the polytopic 1,3,5-triethynylbenzene linker. As suggested in other studies,14 factors such as chain termination by halogen atoms and residual halogen impurities, variable chain length, incomplete metalation and, possibly, homocoupling between the polytopic linker units can lead to a discrepancy between the elemental analysis values and those expected based on the stoichiometry of copolymerization. This is observed in this case and also for 1-Co and 1-Cr. In particular, this may result in a significant relative deviation with regards to the proposed N content as it is present in small proportions relative to C. Nevertheless, the ICP, EDX and XPS analyses (vide infra) confirmed the expected metal content and the expected N/metal ratio.
Synthesis of 1-Co
In a 150 ml oven-dried round bottom flask, a solution of N,N′-bis(5-bromosalicylaldehyde)cyclohexanediimine (0.96 g, 2 mmol) and CoBr2 (0.438 g, 2 mmol) in dry DMF (30 ml, stored over CaH2) was heated at 110 °C under Ar for 30 min. It was cooled to afford a deep green solution. To the solution of the metallated salen ligand, dry triethylamine (6 ml) was added causing a change of color to deep red. The flask was evacuated/backfilled with Ar twice, and the mixture bubbled with Ar for 30 min. Then, 1,3,5-triethynylbenzene (0.3 g, 2 mmol), PdCl2(PPh3)2 (44 mg, 0.063 mmol), and CuI (12 mg, 0.063 mmol) were added in one portion, the flask was evacuated/backfilled with Ar three times, sealed with septum and heated at 80 °C for 48 h with vigorous stirring. It was cooled, filtered and the solid was washed sequentially with DMF, water, THF, MeOH and acetone, then suspended in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CH2Cl2/H2O/MeOH/acetone (160 ml), and stirred at 80 °C for 20 h under air. It was filtered, washed with acetone and dried at high vacuum at 60 °C overnight to produce dark red solid, 1.35 g. Calc. for C33H24N2O2CoBr: C, 63.99%; H, 3.91%; Br, 12.9%; Co, 9.51%; N, 4.52%; O, 5.17%. Found C, 55.58%; H, 3.48%; N, 4.00%.
1 CH2Cl2/H2O/MeOH/acetone (160 ml), and stirred at 80 °C for 20 h under air. It was filtered, washed with acetone and dried at high vacuum at 60 °C overnight to produce dark red solid, 1.35 g. Calc. for C33H24N2O2CoBr: C, 63.99%; H, 3.91%; Br, 12.9%; Co, 9.51%; N, 4.52%; O, 5.17%. Found C, 55.58%; H, 3.48%; N, 4.00%.
Synthesis of 1-Cr
In a 150 ml oven-dried round bottom flask, a solution of N,N′-bis(5-bromosalicylaldehyde)cyclohexanediimine (0.96 g, 2 mmol) and CrCl2 (0.246 g, 2 mmol) in dry DMF (30 ml, stored over CaH2) was heated at 110 °C under Ar for 30 min. It was cooled to afford dark brown solution. To the solution of the metallated salen ligand, dry triethylamine (6 ml) was added, the flask was evacuated/backfilled with Ar twice, and the mixture was bubbled with Ar for 30 min. Then, 1,3,5-triethynylbenzene (0.3 g, 2 mmol), PdCl2(PPh3)2 (44 mg, 0.063 mmol), and CuI (12 mg, 0.063 mmol) were added in one portion and the same synthetic procedure and work-up as for 1-Co was followed to give green brown solid, 0.6 g. Calc. for C33H24N2O2CrCl: C, 69.78%; H, 4.26%; Cl, 6.24%; Cr, 9.15%; N, 4.93%; O, 5.63%. Found C, 68.16%; H, 3.90%; N, 2.44%.Catalysis studies
In a typical setup 1-Cr (50 mg, 0.1 mmol of Cr, 0.2 mol%), NBu4Br (73.2 mg, 0.2 mmol, 0.4 mol%) and propylene oxide (PO) (3.5 ml, 50 mmol) were charged in a 100 ml stainless steel autoclave and CO2 was added to the system until an internal pressure of 16 bar was reached. The reactor was heated up to 100 °C, with the internal pressure stabilized at 20 bar (initial pressure). The mixture was stirred for 16 hours. After this period the system was allowed to cool to room temperature. The reaction vessel was cooled down with ice and the pressure was slowly released. The crude mixture was analyzed by NMR to determine propylene oxide conversion by comparison between the integrals of the corresponding protons of the starting epoxide and the carbonate product as in ref. 4a. The catalyst was recovered by decanting the liquid phase and subsequently suspended in dichloromethane. Dichloromethane was decanted and the catalyst was washed again with fresh dichloromethane. Decantation of the solvent afforded a solid that was dried in an oven at 80 °C for 8 hours. The amount of catalyst recovered for each cycle corresponded to 94–96% of the initial amount.3. Results and discussion
We utilized the Sonogashira–Hagihara cross-coupling reaction to construct POPs through linkage of the tailored ditopic salen molecules containing two bromophenyl groups and the tritopic linker 1,3,5-triethynylbenzene (Scheme 1). The cross-coupling reaction of the non-metalated dibromosalen with 1,3,5-triethynylbenzene resulted in an amorphous, porous solid (1). The metalated salen solutions (cobalt and chromium, respectively) were prepared in situ prior to Sonogashira coupling. The cobalt-metalated salen POP (1-Co) was obtained as deep-red crystalline, porous solid under similar reaction conditions. Accordingly, chromium dibromosalen afforded the chromium-metalated salen POP (1-Cr) as a brown porous solid.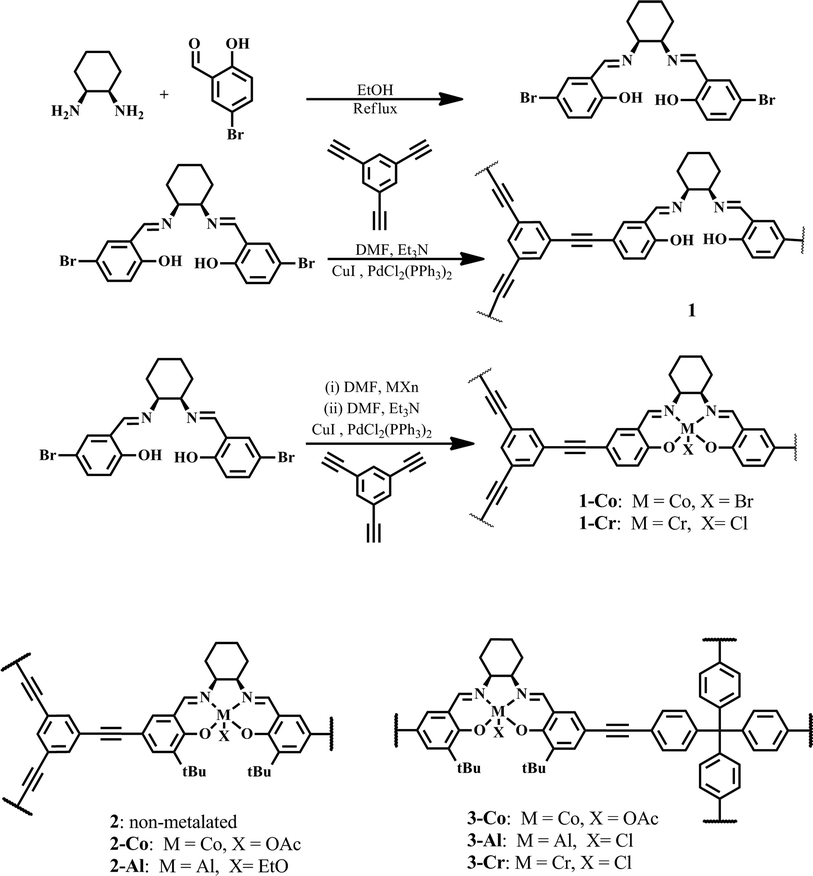 | ||
| Scheme 1 Synthesis of 1, 1-Co and 1-Cr and comparison with other metalosalen-based POPs (2-Co and 2-Al, ref. 12a and 3-Co, 3-Al and 3-Cr, ref. 12b). | ||
SEM images of 1-Co indicated smoother surface morphology as compared to the surface of 1-Cr, further supporting the micro-crystallinity observed for 1-Co as compared to the largely amorphous nature of 1-Cr inferred from X-ray powder diffraction (for PXRD data, see Fig. S1, ESI†). Additionally, both polymers show uniform particle dispersity as shown in Fig. S12, ESI.† The solids were insoluble in water and common organic solvents. Thermogravimetric analysis of all three materials showed loss of weight taking place only above 300 °C, thus confirming the robustness of the compounds (Fig. S3, ESI†).
The N2 sorption isotherms (Fig. 1 and S5, ESI†) of 1, 1-Co, and 1-Cr confirmed their permanent porosity (the measured BET surface areas are 523 m2 g−1 for 1, 370 m2 g−1 for 1-Co and 732 m2 g−1 for 1-Cr). A comparison with the POPs reported by Xie et al.12a (Scheme 1) shows relatively lower surface areas of our materials. In particular, the non-metalated compounds 1 and 2 display a noticeable difference (772 m2 g−1 for 2versus 523 m2 g−1 for 1) that could be attributed to the steric influence of the tert-butyl groups in 2.
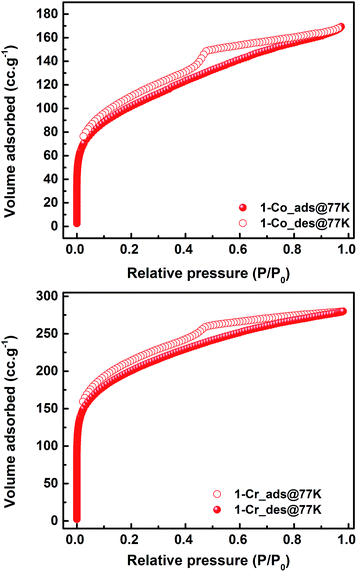 | ||
| Fig. 1 N2 sorption isotherms of 1-Co (370 m2 g−1), and for 1-Cr (732 m2 g−1) confirming the porous nature of the solids. | ||
Interestingly, notable changes in the surface area were noticed for the analogous structures metalated with Co (772 m2 g−1 and 965 m2 g−1 for 2 to 2-Co, and 523 m2 g−1 and 370 m2 g−1 for 1 to 1-Co), indicating significant effect of the starting metal complex on the structural properties of resulting POPs. 1-Co is a crystalline material, as compared to amorphous 1 and 1-Cr, see Fig. 1, ESI.† Finally, 1-Cr and 2-Cr, and 1-Co and 2-Co display surface areas comparable to the POPs prepared by Chun et al.12b (650 m2 g−1 for 3-Al, 580 m2 g−1 for 3-Co and 522 m2 g−1 for 3-Cr, Scheme 1) with the use of tetrahedral linker, tetra(4-ethynylphenyl)methane and in the presence of tert-butyl groups on the salen scaffold.
This shows that the final surface area of the metalated porous solids is strongly dependent on the metal and on the pattern of substitution at the salen scaffold.
The successful salen metalation in 1-Co and 1-Cr is indicated by the observed solid-state UV-Vis (Fig. S4, ESI†) and FT-IR (Fig. S2, ESI†) spectra of the fully washed solids. The characteristic absorption bands for the O–H stretching (phenol, ν = 3294 cm−1) was significantly reduced following metalation in both 1-Co and 1-Cr, indicating deprotonation of the phenolic hydroxyl groups. The UV-Vis spectra for 1-Co and 1-Cr exhibit typical absorption bands at λmax of 486 and ∼400 nm, respectively, characteristic for the metal salen complexes.15
ICP-OES analysis confirmed a Co loading of 8.05 wt% (calculated theoretical maximum loading is 9.51 wt%) and a Cr loading of 7.12 wt% (calculated theoretical maximum loading is 9.15 wt%). These results were further supported by EDX analysis of the polymers, see Fig. S13–S15, ESI.† The difference between the theoretical and experimental loading may be attributed to the incomplete metalation of some inaccessible salen's coordination sites at the preparation stage, or competing coordination of the metal ions by the Et3N base used in the synthesis, and consistent with the observation of a residual OH stretching signal at 3294 cm−1 in the FT-IR spectra (Fig. S2, ESI†). The XPS spectra of 1-Co and 1-Cr display the signals relative to the 2p3/2 orbitals of the metal centers at 779.5 eV and at 576.6 eV respectively. According to the available XPS literature data for Cr16 and Co,17 the metals were found to be in a formal +3 oxidation state (see Fig. S7–S10, ESI,† for a more detailed explanation of the XPS spectra of both compounds). The N/metal ratio of ca. 2 found for 1-Cr and 1-Co from the XPS survey spectra in Fig. S7 and S9† respectively is in a good agreement with the proposed structures and confirms the successful metalation of the salen scaffolds.
Homogenous Al–, Mn–, Cr–, Co– and Zn–salen complexes generally show high catalytic activities towards the cycloaddition of epoxides and CO2 under mild reaction conditions.11 Nevertheless, heterogeneous and recyclable metalosalen catalysts obtained by tethering the catalytic moieties to the traditional solid supports have proven far less active as compared to their soluble counterparts. These results are rationalized as an effect of the immobilization and limited accessibility of the catalytically active core.18 Among the heterogeneous systems showing significant activity under mild conditions,18b,19 MOFs20 and porous organic polymers12,21 have emerged as valuable catalysts for the cycloaddition of CO2 and epoxides in virtue of the high accessibility of the catalytically active centers.
In this study, catalysts 1-Co and 1-Cr showed both remarkable activities towards the formation of cyclic carbonates under mild reaction conditions (see Table 1) in the presence of two equivalent amounts of tetrabutylammonium bromide (NBu4Br) as a nucleophilic co-catalyst. Very high to nearly quantitative conversions were obtained for mono-substituted epoxides 4a–4c (Table 1, entries 5, 7, 9 for 1-Cr; entries 15, 17, 19 for 1-Co) at 100 °C under 20 bar of CO2. Epoxide 2c proved to be the most reactive substrate for this reaction (Table 1, entries 9, 19); nearly quantitative yields of the corresponding carbonate product were obtained after 7 h even when using only half amount of the catalyst employed for epoxides 4a and 4b. Disubstituted epoxide 4d (Table 1, entries 11, 21) afforded only low yields of the corresponding product of cycloaddition as an effect of the increased steric bulk. The catalysts were recovered and reused for three additional catalytic cycles for each of 4a–4c substrates showing outstanding recyclability; the small drop in conversions observed can be mostly attributed to some catalyst loss due to handling at the recovery stage (Table 1, entries 6, 8, 10 for 1-Cr, entries 16, 18, 20 for 1-Co). Leaching of metal species was negligible as confirmed by ICP-OES analysis. Under the same reaction conditions, the metal-free polymer 1 showed much lower catalytic activity for the conversion of epoxide 4a (34% compared to 98% observed using 1-Cr and 89% observed for 1-CoTable 1, entries 2, 5, and 15 for the reaction at 100 °C and 20 bar CO2). This can be attributed to the catalytic activity of the free phenol groups as reported by other authors.22 The NBu4Br co-catalyst, used in the absence of POPs, showed residual catalytic activity both at 22 °C and at 100 °C (Table 1, entry 1) but much lower than when used in combination with 1-Co and 1-Cr under identical reaction conditions.
| Entry | R′′ | Time (h) | Temp. (°C) | Conversiona (%) | TONb | TOF (h−1) | Entry | R′′ | Time (h) | Temp. (°C) | Conversiona (%) | TONb | TOF (h−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Catalyst: 1-Cr)c,d | (Catalyst: 1-Co)i,j | ||||||||||||
| a Determined by 1H NMR (in CDCl3) on an aliquot of the final reaction mixture by comparison of the intensities of signals relative to the corresponding protons for the starting material and the product. b mmol of product/mmol of Cr or Co employed. c Catalyst 1-Cr (50 mg, 0.0685 mmol Cr), NBu4Br (73.2 mg, 0.2 mmol), propylene oxide (3.5 ml, 50 mmol) in a stainless steel autoclave. d Catalyst 1-Cr (100 mg, 0.137 mmol Cr), NBu4Br (146.5 mg, 0.4 mmol), propylene oxide (3.5 ml, 50 mmol). e Control experiments for the NBu4Br co-catalyst carried out using propylene oxide (3.5 ml, 50 mmol) and NBu4Br (549.6 mg, 1.5 mmol) for the experiment at 1 bar CO2, 22 °C in 48 h, or using NBu4Br (73.2 mg, 0.2 mmol) for the experiment at 100 °C, 20 bar CO2 in 6 h (the values for this experiment are given in brackets). f Control experiments for the non-metalated species 1 using: propylene oxide (3.5 ml, 50 mmol) and: 1 (100 mg), NBu4Br (146.5 mg, 0.4 mmol) for the experiment at 1 bar CO2, 22 °C in 48 h, or 1 (50 mg), NBu4Br (73.2 mg, 0.2 mmol) for the experiment at 20 bar CO2, 100 °C in 16 h (the values for this experiment are given in brackets). g At 1 bar CO2. h Reusing the catalyst recycled from the previous entry for three additional catalytic cycles. i Catalyst 1-Co (50 mg, 0.0685 mmol Co), NBu4Br (62.3 mg, 0.17 mmol), propylene oxide (3.5 ml, 50 mmol). j Catalyst 1-Co (72 mg, 0.1 mmol Co), NBu4Br (124.6 mg, 0.34 mmol), propylene oxide (3.5 ml, 50 mmol). k Catalyst 1-Co (100 mg, 0.137 mmol Co), NBu4Br (934.2 mg, 2.55 mmol), propylene oxide (3.5 ml, 50 mmol). | |||||||||||||
| 1e | Me | 48 (6) | 22 (100) | 5 (14) | 12j,g | Me | 48 | 22 | 25 | 125 | 3 | ||
| 2f | Me | 48 (16) | 22 (100) | 8 (34) | 13k,g | Me | 48 | 22 | 26 | 130 | 3 | ||
| 3c,g | Me | 48 | 22 | 19 | 139 | 3 | 14j | Me | 16 | 75 | 61 | 223 | 14 |
| 4d | Me | 16 | 75 | 68 | 248 | 15 | 15j | Me | 16 | 100 | 89 | 325 | 20 |
| 5d | Me | 16 | 100 | 98 | 358 | 22 | 16j,h | Me | 16 | 100 | 88, 83, 79 | ||
| 6d,h | Me | 16 | 100 | 95, 90, 85 | 17j | Ph | 6 | 100 | 97 | 354 | 59 | ||
| 7d | Ph | 6 | 100 | 82 | 299 | 50 | 18j,h | Ph | 6 | 100 | 85, 82, 79 | ||
| 8d,h | Ph | 6 | 100 | 75, 70, 69 | 19i | CH2Cl | 7 | 100 | 92 | 672 | 96 | ||
| 9c | CH2Cl | 7 | 100 | 95 | 693 | 99 | 20i,h | CH2Cl | 7 | 100 | 88, 82, 79 | ||
| 10c,h | CH2Cl | 7 | 100 | 90, 87, 83 | 21i | –(CH2)4– | 6 | 100 | 20 | 146 | 24 | ||
| 11c | –(CH2)4– | 6 | 100 | 28 | 204 | 34 | |||||||
Further investigation of the activity of 1-Co and 1-Cr was dedicated to their catalytic performance under even milder conditions. When the reaction was performed at 75 °C, moderate to good conversions were obtained for both catalysts (Table 1, entries 4 and 14). Then, 1-Co and 1-Cr were tested for the reaction between 4a and CO2 under ambient conditions (25 °C, 1 bar CO2). The catalysts proved to be active even under such challenging reaction conditions, although only low yields of the corresponding carbonate were obtained after 48 h (19% and 25% for 1-Cr and for 1-Co respectively, Table 1, entries 3 and 12). A comparison between 1-Co and the analogous Co salen-based microporous polymer developed by Xie et al.12a (2-Co) and employed under comparable reaction conditions, but using up to 15 equiv. of NBu4Br per mole of metal as a co-catalyst for 48 h, shows a slightly higher TON (167 vs. 125) in favor of the latter POP. In our case, increasing the NBu4Br/Co molar ratio from 2 to 15 did not have any substantial effect on the catalytic performance (Table 1, entry 13). The difference in activity under ambient conditions, observed for 2-Co (Scheme 1), and our materials can be explained on the basis of a combination of both electronic and structural effects. Indeed, complexes 1-Co and 2-Co differ for the presence of t-butyl substituents at the aromatic ligands and for the counter-ions of the cobalt cations (Br− in our work, AcO− for Xie work). It is well known that both factors can have a strong influence for tuning the catalytic activity of the salen complex metal centers, by altering its Lewis acidity.11,23 This is known to have a strong impact on the interaction between the nucleophilic co-catalyst and the metal center, on the ability of the latter to activate the epoxide for ring opening and on the following steps of CO2 insertion and release of the carbonate product via cyclization. The barrier for these steps is known to depend on a fine balance between the Lewis acidity of the metal center and the nucleophilicity and basicity of the co-catalyst.4b,24 In this context, the presence of electron donating t-butyl substituents at the aromatic rings has been found to have a dramatic effect on the yield of the cycloaddition reaction of CO2 to epoxides catalyzed by Co(III),25 Al,26 Zn,27 and Cu26 salen complexes. The direction of this effect (i.e. towards higher or lower catalytic efficiency) depends on the metal center. In the case of homogeneous Co(III) salen catalysts, the presence of t-butyl substituents at the aromatic rings was found to have the effect of a strong increase in catalytic efficiency as compared to the analogous Co(III) complex with unsubstituted aromatic rings.25 This is in a good agreement with what was observed for catalysts 1-Co and 2-Co. The presence of the AcO− counter-ion in 2-Co could also play a role in increasing its catalytic activity versus1-Co according to a comparative study for soluble Co(III) salen complexes.25 However, on the basis of the results obtained for the homogeneous complexes, this effect is expected to be less pronounced than that of the substitution pattern at the phenyl rings of the scaffold. Assuming that the catalytic reaction takes place also within the pores of the POPs and not just on their exposed surface, the larger surface area of 2-Co (965 m2 g−1) compared to 1-Co (370 m2 g−1) might also play a significant role in ensuring higher accessibility of the active centers.
Remarkably, POPs 3-Cr and 3-Co,12b having respectively lower and larger surface area as compared to those of 1-Co and 1-Cr (and in the case of 3-Co also a different counter-anion) displayed both higher TON values for the conversion of 4a than 1-Co and 1-Cr (cf. in particular the performance of 1-Co, 1-Cr at 75 °C, Table 1, entries 4, 14 and the results reported by Chun et al. at 60 °C in ref. 12b). Taken together, these results indicate that the electronic effects generated by the substitution pattern of the salen scaffold may have a stronger influence on the catalytic activity than the surface area of the polymer.
We compared the catalytic performance of 1-Co and 1-Cr with that of other supported salen systems. Interestingly, the conversion of epoxide 4a afforded by our catalysts in 16 h at 100 °C and 20 bar CO2 (Table 1, entries 5, 15) is higher than that reported by Jiang et al. under comparable conditions (100 °C, 20 bar, 24 h) for a Ni(salphen) catalyst supported within a Cd-based MOF.20k The activity of the latter system is higher at slightly lower temperatures as it affords 80% 4a conversion in 4 h at 80 °C and 20 bar CO2 (see also Table 1, entries 4 and 14) but it is not reported under ambient conditions. Amongst the salen-based heterogeneous catalysts active under ambient conditions for the title reaction, it is worth mentioning a silica-supported single component Al–salen bimetallic catalyst by North et al.18b This system affords 86% 4a conversion in 24 h (TOF = 0.7) at 26 °C and 1 bar CO2 using a high catalyst loading (5 mol% Al versus 0.137 mol% Cr for 1-Cr, Table 1, entry 3 and 0.2 mol% Co for 1-Co, Table 1, entry 12). However, it does not require the addition of external nucleophiles. The preparation of such single component POPs for the cycloaddition of CO2 to epoxides is highly desirable in order to fully exploit the potential of this novel catalyst design.
4. Conclusions
We report the application of novel POPs as heterogeneous catalysts for CO2 conversion, and specifically, as porous solid matrices where catalytically active molecules are covalently integrated into the scaffold backbone. In the presented metalosalen-based POPs, the coupled enhancement of catalyst lifetime and facile recyclability confirms the potential of POPs as novel matrices to immobilize small functional molecules into porous robust solids. The obtained catalytic results are compared with those reported for other salen-based POPs that have been employed as catalysts for the title reaction. It is worth highlighting that analogously as for the homogeneous catalysis, the electronic features of the salen backbone play a decisive role in catalysis, and can be amplified by the morphological properties of the synthesized POPs. In the light of the nearly infinite number of possibilities in terms of the choice of the salen scaffold, metal, counter-anion and polytopic linker, very active heterogeneous catalysts can be generated in the future for the fixation of CO2, by its cycloaddition to epoxides to yield liquid organic carbonates.Acknowledgements
Research reported in this publication was supported by the King Abdullah University of Science and Technology (KAUST).Notes and references
- (a) Z.-Z. Yang, L.-N. He, J. Gao, A.-H. Liu and B. Yu, Energy Environ. Sci., 2012, 5, 6602 RSC; (b) S. H. Kim, K. H. Kim and S. H. Hong, Angew. Chem., Int. Ed., 2014, 53, 771 CrossRef CAS PubMed.
- (a) I. Omae, Coord. Chem. Rev., 2012, 256, 1384 CrossRef CAS; (b) M. Cokoja, C. Bruckmeier, B. Rieger, W. A. Herrmann and F. E. Kühn, Angew. Chem., Int. Ed., 2011, 50, 8510 CrossRef CAS PubMed; (c) Carbon Dioxide as Chemical Feedstock, ed. M. Aresta, Wiley-VCH, Weinheim, 2010 Search PubMed; (d) M. Mikkelsen, M. Jørgensen and F. C. Krebs, Energy Environ. Sci., 2010, 3, 43 RSC; (e) S. Y. T. Lee, A. A. Ghani, V. D'Elia, M. Cokoja, W. A. Herrmann, J.-M. Basset and F. E. Kühn, New J. Chem., 2013, 37, 3512 RSC.
- (a) M. O. Sonnati, S. Amigoni, E. P. Taffin de Givenchy, T. Darmanin, O. Choulet and F. Guittard, Green Chem., 2013, 15, 283 RSC; (b) X.-B. Lu and D. J. Darensbourg, Chem. Soc. Rev., 2012, 41, 1462 RSC; (c) P. P. Pescarmona and M. Taherimehr, Catal. Sci. Technol., 2012, 2, 2169 RSC; (d) M. North, R. Pasquale and C. Young, Green Chem., 2010, 12, 1514 RSC; (e) B. Schäffner, F. Schäffner, S. P. Verevkin and A. Börner, Chem. Rev., 2010, 110, 4554 CrossRef PubMed; (f) T. Sakakura and K. Kohno, Chem. Commun., 2009, 1312 RSC.
- (a) A. Monassier, V. D'Elia, M. Cokoja, H. Dong, J. D. A. Pelletier, J.-M. Basset and F. E. Kühn, ChemCatChem, 2013, 5, 1321 CrossRef CAS; (b) M. E. Wilhelm, M. H. Anthofer, R. M. Reich, V. D'Elia, J.-M. Basset, W. A. Herrmann, M. Cokoja and F. E. Kühn, Catal. Sci. Technol., 2014, 4, 1638 RSC; (c) B. Dutta, J. Sofack-Kreutzer, A. A. Ghani, V. D'Elia, J. D. A. Pelletier, M. Cokoja, F. E. Kühn and J.-M. Basset, Catal. Sci. Technol., 2014, 4, 1534 RSC; (d) V. D'Elia, A. A. Ghani, A. Monassier, J. Sofack-Kreutzer, J. D. A. Pelletier, M. Drees, S. V. C. Vummaleti, A. Poater, L. Cavallo, M. Cokoja, J.-M. Basset and F. E. Kühn, Chem.–Eur. J., 2014, 20, 11870 CrossRef PubMed; (e) V. D'Elia, J. D. A. Pelletier and J.-M. Basset, ChemCatChem, 2015, 7, 1906 CrossRef; (f) A. Barthel, Y. Saih, M. Gimenez, J. D. A. Pelletier, F. E. Kühn, V. D’Elia and J. M. Basset, Green Chem., 2016 10.1039/C5GC03007B.
- (a) W.-Y. Gao, Y. Chen, Y. Niu, K. Williams, L. Cash, P. J. Perez, L. Wojtas, J. Cai, Y.-S. Chen and S. Ma, Angew. Chem., Int. Ed., 2014, 53, 2615 CrossRef CAS PubMed; (b) H. V. Babu and K. Muralidharan, Dalton Trans., 2013, 42, 1238 RSC; (c) C. J. Whiteoak, E. Martin, M. M. Belmonte, J. Benet-Buchholz and A. W. Kleij, Adv. Synth. Catal., 2012, 354, 469 CrossRef CAS; (d) J. A. Castro-Osma, A. L. Sanchez, M. North, A. Otero and P. Villuendas, Catal. Sci. Technol., 2012, 2, 1021 RSC; (e) Y. Yang, Y. Hayashi, Y. Fujii, T. Nagano, Y. Kita, T. Ohshima, J. Okuda and K. Mashima, Catal. Sci. Technol., 2012, 2, 509 RSC; (f) A. Buchard, M. R. Kember, K. G. Sandeman and C. K. Williams, Chem. Commun., 2011, 47, 212 RSC; (g) W. Clegg, R. W. Harrington, M. North and R. Pasquale, Chem.–Eur. J., 2010, 16, 6828 CrossRef CAS PubMed; (h) S. F. Yin and S. Shimada, Chem. Commun., 2009, 1136 RSC; (i) J. Meléndez, M. North and R. Pasquale, Eur. J. Inorg. Chem., 2007, 3323 CrossRef.
- For recent reviews, see: (a) U. H. F. Bunz, K. Seehafer, F. L. Geyer, M. Bender, I. Braun, E. Smarsly and J. Freudenberg, Macromol. Rapid Commun., 2014, 35, 1466 CrossRef CAS PubMed; (b) M. L. Foo, R. Matsuda and S. Kitagawa, Chem. Mater., 2014, 26, 310 CrossRef CAS; (c) Q. Liu, Z. Tang, M. Wu and Z. Zhou, Polym. Int., 2014, 63, 381 CrossRef CAS; (d) X. Zou, H. Rena and G. Zhu, Chem. Commun., 2013, 49, 3925 RSC; (e) Y. Xu, S. Jin, H. Xu, A. Nagai and D. Jiang, Chem. Soc. Rev., 2013, 42, 8012 RSC; (f) S. Xu, Y. Luo and B. Tan, Macromol. Rapid Commun., 2013, 34, 471 CrossRef CAS PubMed; (g) R. Dawson, A. I. Cooper and D. J. Adams, Prog. Polym. Sci., 2012, 37, 530 CrossRef CAS.
- (a) Z. Chang, D.-S. Zhang, Q. Chen and X.-H. Bu, Phys. Chem. Chem. Phys., 2013, 15, 5430 RSC; (b) F. Vilela, K. Zhang and M. Antonietti, Energy Environ. Sci., 2012, 5, 7819 RSCfor selected recent examples, see: (c) H. Li, Z. Li, Y. Zhang, X. Luo, H. Xia, X. Liu and Y. Mua, RSC Adv., 2014, 4, 37767 RSC; (d) S. J. Ren, R. Dawson, A. Laybourn, J. X. Jiang, Y. Khimyak, D. J. Adams and A. I. Cooper, Polym. Chem., 2012, 3, 928 RSC; (e) R. Dawson, D. J. Adams and A. I. Cooper, Chem. Sci., 2011, 2, 1173 RSC; (f) W. Lu, D. Y. D. Zhao, C. I. Schilling, O. Plietzsch, T. Muller, S. Bräse, J. Guenther, J. Blümel, R. Krishna, Z. Li and H.-C. Zhou, Chem. Mater., 2010, 22, 5964 CrossRef CAS; (g) S. W. Yuan, B. Dorney, D. White, S. Kirklin, P. Zapol, L. P. Yu and D. J. Liu, Chem. Commun., 2010, 46, 4547 RSC; (h) J. Chun, S. Kang, N. Park, E. J. Park, X. Jin, K.-D. Kim, H. O. Seo, S. M. Lee, H. J. Kim, W. H. Kwon, Y.-K. Park, J. M. Kim, Y. D. Kim and S. U. Son, J. Am. Chem. Soc., 2014, 136, 6786 CrossRef CAS PubMed.
- For selected recent examples, see: (a) C. Gu, Y. C. Chen, Z. B. Zhang, S. F. Xue, S. H. Sun, K. Zhang, C. M. Zhong, H. H. Zhang, Y. Y. Pan, Y. Lv, Y. Q. Yang, F. H. Li, S. B. Zhang, F. Huang and Y. G. Ma, Adv. Mater., 2013, 25, 3443 CrossRef CAS PubMed; (b) Y. Xu, A. Nagai and D. Jiang, Chem. Commun., 2013, 49, 1591 RSC; (c) Y. H. Xu, L. Chen, Z. Q. Guo, A. Nagai and D. Jiang, J. Am. Chem. Soc., 2011, 133, 17622 CrossRef CAS PubMed; (d) J.-X. Jiang, A. Trewin, D. J. Adams and A. I. Cooper, Chem. Sci., 2011, 2, 1777 RSC; (e) L. Chen, Y. Honsho, S. Seki and D. Jiang, J. Am. Chem. Soc., 2010, 132, 6742 CrossRef CAS PubMed.
- (a) X. Liu, S. A. Y. Zhang, X. Luo, H. Xia, H. Lia and Y. Mu, RSC Adv., 2014, 4, 6447 RSC; (b) N. Kang, J. H. Park, M. Jin, N. Park, S. M. Lee, H. J. Kim, J. M. Kim and S. U. Son, J. Am. Chem. Soc., 2013, 135, 19115 CrossRef CAS PubMed; (c) A. Modak, M. Pramanik, S. Inagaki and A. Bhaumik, J. Mater. Chem. A, 2014, 2, 11642 RSC; (d) W.-K. An, M.-Y. Han, C.-A. Wang, S.-M. Yu, Y. Zhang, S. Bai and W. Wang, Chem.–Eur. J., 2014, 20, 11019 CrossRef CAS PubMed; (e) J. Schmidt, D. S. Kundu, S. Blechert and A. Thomas, Chem. Commun., 2014, 50, 3347 RSC; (f) L. Li, H. Zhao, J. Wang and R. Wang, ACS Nano, 2014, 8, 5352 CrossRef CAS PubMed; (g) H. Li, B. Xu, X. Liu, S. A. C. He, H. Xia and Y. Mu, J. Mater. Chem. A, 2013, 1, 14108 RSC.
- (a) C. Baleizao and H. Garcia, Chem. Soc. Rev., 2006, 106, 3987 CrossRef CAS PubMed; (b) P. G. Cozzi, Chem. Rev., 2004, 33, 410 RSC.
- A. Decortes, A. M. Castilla and A. W. Kleij, Angew. Chem., Int. Ed., 2010, 49, 9822 CrossRef CAS PubMed.
- (a) Y. Xie, T.-T. Wang, X.-H. Liu, K. Zou and W.-Q. Deng, Nat. Commun., 2013, 4, 1960 Search PubMed; (b) J. Chun, S. Kang, N. Kang, S. M. Lee, H. J. Kim and S. U. Son, J. Mater. Chem. A, 2013, 1, 5517 RSC.
- A. R. Silva, T. Mourão and J. Rocha, Catal. Today, 2013, 81 CrossRef CAS.
- V. Guillerm, Ł. J. Weseliński, M. H. Alkordi, M. I. H. Mohideen, Y. Belmabkhout, A. J. Cairns and M. Eddaoudi, Chem. Commun., 2014, 50, 1937 RSC.
- K. P. Bryliakov and E. P. Talsi, Inorg. Chem., 2003, 42, 7258 CrossRef CAS PubMed.
- (a) I. Grohmann, E. Kemnitz, A. Lippitz and W. E. S. Unger, Surf. Interface Anal., 1995, 23, 887 CrossRef CAS; (b) T. Gross, D. Treu, E. Ünveren, E. Kemnitz and W. E. S. Unger, Surf. Sci. Spectra, 2008, 15, 77 CrossRef; (c) M. Aronniemi, J. Sainio and J. Lahtinen, Surf. Sci., 2005, 578, 108 CrossRef CAS.
- (a) T. J. Chuang, C. R. Bridle and D. W. Rice, Surf. Sci., 1976, 59, 413 CrossRef CAS; (b) C. A. F. Vaz, D. Prabhakaran, E. I. Altman and V. E. Henrich, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 155457 CrossRef.
- (a) W. L. Dai, S. L. Luo, S. F. Yin and C. T. Au, Appl. Catal., A, 2009, 366, 2 CrossRef CAS; (b) J. Melendez, M. North, P. Villuendas and C. Young, Dalton Trans., 2011, 40, 3885 RSC; (c) M. North, P. Villuendas and C. Young, Chem.–Eur. J., 2009, 15, 11454 CrossRef CAS PubMed; (d) F. Jutz, J. D. Grunwaldt and A. Baiker, J. Mol. Catal. A: Chem., 2008, 279, 94 CrossRef CAS; (e) M. Ramin, F. Jutz, J. D. Grunwaldt and A. Baiker, J. Mol. Catal. A: Chem., 2005, 242, 32 CrossRef CAS; (f) M. Alvaro, C. Baleizao, E. Carbonell, M. El Ghoul, H. Garcia and B. Gigante, Tetrahedron, 2005, 61, 12131 CrossRef CAS; (g) M. Alvaro, C. Baleizao, D. Das, E. Carbonell and H. Garcia, J. Catal., 2004, 228, 254 CrossRef CAS; (h) X. B. Lu, J. H. Xiu, R. He, K. Jin, L. M. Luo and X. J. Feng, Appl. Catal., A, 2004, 275, 73 CrossRef CAS.
- (a) J. Meléndez, M. North and P. Villuendas, Chem. Commun., 2009, 2577 RSC; (b) V. D'Elia, H. Dong, A. J. Rossini, C. M. Widdifield, S. V. C. Vummaleti, Y. Minenkov, A. Poater, E. Abou-Hamad, J. D. A. Pelletier, L. Cavallo, L. Emsley and J.-M. Basset, J. Am. Chem. Soc., 2015, 137, 7728 CrossRef PubMed.
- (a) V. Guillerm, Ł. J. Weseliński, Y. Belmabkhout, A. J. Cairns, V. D'Elia, L. Wojtas, K. Adil and M. Eddaoudi, Nat. Chem., 2014, 6, 673 CAS; (b) A. C. Kathalikkattil, D.-W. Kim, J. Tharun, H.-G. Soek, R. Roshan and D.-W. Park, Green Chem., 2014, 16, 1607 RSC; (c) A. C. Kathalikkattil, R. Roshan, J. Tharun, H. G. Soek, H.-S. Ryu and D.-W. Park, ChemCatChem, 2014, 6, 284 CrossRef CAS; (d) X. Huang, Y. Chen, Z. Lin, X. Ren, Y. Song, Z. Xu, X. Dong, X. Li, C. Hu and B. Wang, Chem. Commun., 2014, 50, 2624 RSC; (e) D. Feng, W.-C. Chung, Z. Wei, Z.-Y. Gu, H.-L. Jiang, Y.-P. Chen, D. J. Darensbourg and H.-C. Zhou, J. Am. Chem. Soc., 2013, 135, 17105 CrossRef CAS PubMed; (f) O. V. Zalomaeva, A. M. Chibiryaev, K. A. Kovalenko, O. A. Kholdeeva, B. S. Balzhinimaev and V. P. Fedin, J. Catal., 2013, 298, 179 CrossRef CAS; (g) Y. Ren, X. Cheng, S. Yang, C. Qi, H. Jiang and Q. Mao, Dalton Trans., 2013, 42, 9930 RSC; (h) X. Zhou, Y. Zhang, X. Yang, L. Zhao and G. Wang, J. Mol. Catal. A: Chem., 2012, 361–362, 12 CrossRef CAS; (i) T. Lescouet, C. Chizallet and D. Farrusseng, ChemCatChem, 2012, 4, 1725 CrossRef CAS; (j) J. Song, Z. Zhang, S. Hu, T. Wu, T. Jiang and B. Han, Green Chem., 2009, 11, 1031 RSC; (k) Y. Ren, Y. Shi, J. Chen, S. Yang, C. Qi and H. Jiang, RSC Adv., 2013, 3, 2167 RSC.
- J. Roeser, K. Kailasam and A. Thomas, ChemSusChem, 2012, 5, 1793 CrossRef CAS PubMed.
- (a) C. J. Whiteoak, A. Nova, F. Maseras and A. W. Kleij, ChemSusChem, 2012, 5, 2032 CrossRef CAS PubMed; (b) Y.-M. Shen, W.-L. Duan and M. Shi, Eur. J. Org. Chem., 2004, 3080 CrossRef CAS.
- (a) L. Chiang, L. E. N. Allan, J. Alcantara, M. C. P. Wang, T. Storr and M. P. Shaver, Dalton Trans., 2014, 43, 4295 RSC; (b) L. Cavallo and H. Jacobsen, J. Org. Chem., 2003, 68, 6202 CrossRef CAS PubMed.
- F. Castro-Gomez, G. Salassa, A. W. Kleij and C. Bo, Chem.–Eur. J., 2013, 19, 6289 CrossRef CAS PubMed.
- T. Chang, H. Jing, L. Jin and W. Qiu, J. Mol. Catal. A: Chem., 2007, 264, 241 CrossRef CAS.
- X.-B. Lu, Y.-J. Zhang, K. Jin, L.-M. Luo and H. Wang, J. Catal., 2004, 227, 537 CrossRef CAS.
- Y.-M. Shen, W.-L. Duan and M. Shi, J. Org. Chem., 2003, 68, 1559 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Materials and methods, synthesis, PXRD, NMR, FTIR, TGA, UV spectroscopy, gas sorption isotherms, detailed XPS study, SEM, and EDX. See DOI: 10.1039/c5ta09321j |
| ‡ These authors contributed equally to this work. |
| § Current address: Zewail City of Science and Technology, Center for Materials Science, Sheikh Zayed Dist., 6th of October, 12588, Giza, Egypt. |
| ¶ Current address: Department of Chemistry, Michigan Technological University, 1400 Townsend Drive, Houghton, Michigan 49931, USA. |
| || Current address: Department of Materials Science and Engineering, Vidyasirimedhi Institute of Science and Technology, 21210, WangChan, Rayong, Thailand. |
| This journal is © The Royal Society of Chemistry 2016 |

