Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fabricating large two-dimensional single colloidal crystals by doping with active particles
B.
van der Meer
*,
L.
Filion
and
M.
Dijkstra
*
Soft Condensed Matter, Debye Institute for Nanomaterials Science, Utrecht University, Princetonplein 5, 3584 CC Utrecht, The Netherlands. E-mail: B.vanderMeer@uu.nl; M.Dijkstra@uu.nl
First published on 22nd February 2016
Abstract
Using simulations we explore the behaviour of two-dimensional colloidal (poly)crystals doped with active particles. We show that these active dopants can provide an elegant new route to removing grain boundaries in polycrystals. Specifically, we show that active dopants both generate and are attracted to defects, such as vacancies and interstitials, which leads to clustering of dopants at grain boundaries. The active particles both broaden and enhance the mobility of the grain boundaries, causing rapid coarsening of the crystal domains. The remaining defects recrystallize upon turning off the activity of the dopants, resulting in a large-scale single-domain crystal.
Introduction
Colloidal crystals are ordered, self-assembled solids comprised of (sub)micron spheres that are arranged on a periodic lattice. Such crystals are almost always polycrystalline in nature and feature grain boundaries that separate adjacent crystal domains of different crystallographic orientations.1 Unfortunately, the presence of these grain boundaries is well known to drastically alter the material properties compared to a single crystal, for instance altering the photonic properties of the crystal.2 Avoiding the formation of grain boundaries during the fabrication of colloidal crystals is very challenging and typically relies on either controlled growth of a single domain,2,3 epitaxial growth from a template,4–7 or applying external fields.8–10 Hence, new methods to remove grain boundaries are highly desirable. Here, we examine an elegant, new avenue for removing grain boundaries: doping colloidal polycrystals with self-propelled particles.Self-propelled particles, also known as active particles, incessantly convert energy into self-propulsion, and as such are intrinsically out-of-equilibrium. While traditionally such particles occurred solely within the purview of natural systems (e.g. bacteria11,12), recent experimental breakthroughs have led to many novel types of synthetic colloidal swimmers and self-propelled particles.13–18 These systems exhibit a wealth of new phase behaviour, including, for example, motility-induced phase separation into dense and dilute phases,16–26 giant density fluctuations,27,28 and swarming.29 Moreover, experimental and simulation studies have shown that the dynamics of a passive system can be altered dramatically by the incorporation of active particles.30–32 Even for very low concentrations (<1%), where the self-propelled particles can be viewed as active “dopants”, they can strongly alter the properties (e.g. dynamics) of the underlying passive system. Specifically, active dopants have been shown to promote crystallization in hard sphere glasses30 and it has recently been suggested that active dopants can be used to help fabricate large, defect-free crystals.31 Here we confirm this possibility using computer simulations of dense mixtures of active and passive spherical colloids, and explore the mechanism responsible for the growth of crystal domains.
Method and model
We perform Brownian dynamics simulations of two-dimensional crystals consisting of N particles interacting with a purely repulsive Weeks–Chandler–Andersen (WCA) potential | (1) |
Note that we have assumed throughout this study that the active particles interact in exactly the same manner as their passive counterparts. This ensures that the active particles are compatible with the underlying crystal of passive particles.
Results
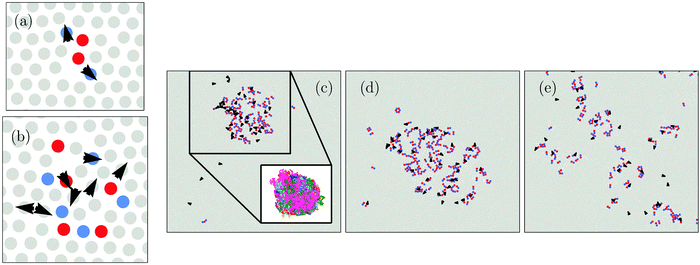
We begin by examining the effect of active dopants on grain boundaries. In Fig. 1 we show a time series of the evolution of a polycrystal in thermal equilibrium (α = 0) and in the case where some particles are active (α = 0.0076). The initial polycrystal, shown in Fig. 1(a), was created by filling polygonal domains with crystallites of different orientations. For the doped case, a random selection of particles were then identified as active. Note that this assumes that the particles are turned active after the crystallization process has taken place. In the snapshots in Fig. 1, we color code particles according to their local orientation usingwith
 the number of nearest neighbours of particle i as determined by a Voronoi construction, and the angle θij = tan−1(rj − ri) with ri the position of particle i and −π ≤ θij ≤ π.33 In Fig. 1(a–e) we show how the passive system evolves in time. Clearly, in thermal equilibrium (α = 0) the grain structure evolves very slowly: the thermal fluctuations allow for some motion of the grain boundaries over long periods of time, yet their mobility is very low, and as such little grain growth occurs. However, as shown in Fig. 1(f–h), upon activation of a small number of particles, α = 0.0076, we observe a large increase in the grain boundary mobility, leading to significant coarsening of the domains over time. Interestingly, whereas in thermal equilibrium the grains are separated by a thin, smooth interface, the grain boundaries in the activated systems are highly fluctuating and much broader, reminiscent of the local melting of the grain surfaces observed in ref. 31. Note that we also examined the evolution of polycrystals that were obtained through crystallization of a supercooled fluid (instead of the artificially formed polycrystals as shown in Fig. 1) and observed no significant differences. The time evolution of such a system is shown in Fig. 2.
the number of nearest neighbours of particle i as determined by a Voronoi construction, and the angle θij = tan−1(rj − ri) with ri the position of particle i and −π ≤ θij ≤ π.33 In Fig. 1(a–e) we show how the passive system evolves in time. Clearly, in thermal equilibrium (α = 0) the grain structure evolves very slowly: the thermal fluctuations allow for some motion of the grain boundaries over long periods of time, yet their mobility is very low, and as such little grain growth occurs. However, as shown in Fig. 1(f–h), upon activation of a small number of particles, α = 0.0076, we observe a large increase in the grain boundary mobility, leading to significant coarsening of the domains over time. Interestingly, whereas in thermal equilibrium the grains are separated by a thin, smooth interface, the grain boundaries in the activated systems are highly fluctuating and much broader, reminiscent of the local melting of the grain surfaces observed in ref. 31. Note that we also examined the evolution of polycrystals that were obtained through crystallization of a supercooled fluid (instead of the artificially formed polycrystals as shown in Fig. 1) and observed no significant differences. The time evolution of such a system is shown in Fig. 2.
From Fig. 1 and 2 it is clear that upon activation, self-propelled particles accumulate at the grain boundaries. This accumulation of self-propelled particles enables the rearrangement of many particles at the surface of the grains leading to significant motion of the boundary over time. In the process, the grain boundaries essentially disappear, and what remains is a single-domain crystal which now contains many local defects as the active particles are constantly perturbing the crystal lattice. The active particles have thus restructured the solid and annealed out the otherwise persistent grain boundaries, at the expense of creating other local defects.
While the removal of grain boundaries is in itself an important step in developing single crystals, it is also highly desirable to remove these local defects. It turns out that this comes almost for free. Specifically, if we switch the activity off, i.e. making all particles passive again (f = 0) [Fig. 1(i)], most of the local defects disappear yielding an almost defect-free single crystal. Importantly, this is not simply a simulation trick, experimentally one may switch off the active particles by using particles that are responsive to certain stimuli (e.g. light-activated colloids) or simply by letting all the fuel be consumed.
An important question that arises in looking at Fig. 1 and 2 is how active particles that are located within a domain migrate to the grain boundaries to mediate the active grain growth. Hence, to better understand the dynamics of the active dopants within the grains, we now focus on the behaviour of active particles in a single crystal. In Fig. 3(a), we plot the trajectories of active particles in such a single crystal domain. We clearly observe two distinct types of trajectories: some active particles migrate large distances over extended periods of time (red trajectories), while other active particles remain caged by their passive neighbours (blue trajectories).
To disentangle this mobile/immobile duality we follow the topological structure of the solid over time using a Voronoi construction which allows us to locate, in addition to the active particles, point defects in the crystals. From studying the topological structure of various snapshots, we observe that the immobile self-propelled particles are trapped inside defect-free hexagonal cages while the mobile self-propelled particles migrate by carrying a vacancy or interstitial. In Fig. 3(b and c) we plot the trajectories of a single active particle interacting with a single vacancy and interstitial, respectively. Note that in the configurations displayed in Fig. 3(b and c) the active particle is highlighted in green and particles with seven-fold and five-fold coordination are shown in red and blue, respectively. Clearly, the active particle and the defects remain in close proximity to each other. The strong overlap between the trajectories suggest that the defect and the active particle are attracted to each other.
Note that these defects form spontaneously due to the activity of the self-propelled particles, even when we start from a perfect single crystal. These defects diffuse along with the active particle as it moves through the crystal, allowing it to remain mobile for long periods of time. Hence, our data illustrate that active particles not only interact very strongly with the grain boundaries, but also with many other types of defects within the crystal such as (multi) vacancies and interstitials. These defects thus play a pivotal role in determining the mobility of the active particles.
To quantify the increase in mobility of self-propelled dopants upon picking up point defects, we consider the mean square displacement 〈Δr2(t)〉 = 〈|r(t) − r(0)|2〉 of a self-propelled particle carrying a vacancy, an interstitial, and no point defect. Self-propelled particles with no defect are caged resulting in a clear plateau in 〈Δr2(t)〉, while self-propelled particles with a defect exhibit substantial diffusion [Fig. 3(d)]. These self-propelled particles with point defects exhibit an intermediate super-diffusive regime, scaling as 〈Δr2(t)〉 ∼ tb with 1 < b ≲ 1.5. Such a super-diffusive regime originates from the ballistic motion inside the cage or during cage jumping. While self-propelled particles with interstitials enter a diffusive regime soon after hopping, when 〈Δr2(t)〉 ≈ σ2, self-propelled particles with vacancies exhibit sub-diffusive motion after having travelled one lattice spacing. This feature arises from the fact that self-propelled particles with a vacancy become trapped inside the vacancy for a while. As a consequence, self-propelled particles have an enhanced probability of rotating their axis of self-propulsion and jumping back into the vacancy, thus leading to a sub-diffusive regime. Eventually, the diffusion mediated by the vacancies also becomes Fickian, scaling as 〈Δr2(t)〉 ∼ t.
The interactions between active particles and crystal defects can also generate attractive interactions between the active particles themselves. For instance, in Fig. 4(a and b), we show a few snapshots where active particles share defects for fσ/kBT = 50 and α = 0.002. Here, the active particles are marked with superimposed arrows that point in the direction of their self-propulsion axis. We observe that single vacancies or interstitials are often shared between two active particles, as shown in Fig. 4(a), leading to attractions.
The attractive interactions between point defects in all combinations (vacancy–vacancy, interstitial–interstitial, and vacancy–interstitial) can lead to the formation of linear defect strings.34,35 We find that active particles typically accumulate at the end of these defect strings and we show one such example in Fig. 4(b). Here many additional defects have formed a small cluster at the end of the string, owing to the fact that the active particles are constantly exciting the end of the defect string. If we increase the fraction of active particles α = 0.004 we observe that these defect clusters grow and eventually coalesce to form one large defect cluster that is rich in active particles, as shown in Fig. 4(c). The attractive interactions between point defects thus drives the system to “phase separate” into a hexagonal crystal and a complex defect cluster which is rich in active particles. We plot the trajectories of some active particles that are inside the big defect cluster in the inset of Fig. 4(c). Clearly, the active particles become trapped inside the defect cluster and are unlikely to leave the defected area. Nonetheless, they can move throughout the whole defect zone, migrating from one side of the cluster to the other. The size of the defect zone increases with increasing self-propulsion [Fig. 4(d)]. However, upon increasing the self-propulsion f further this cluster breaks up, as shown in Fig. 4(e).
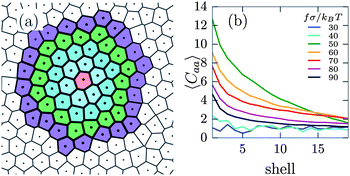
To further explore the attraction between active particles, we determine the fraction of active neighbours of an active particle in concentric Voronoi shells. Specifically, for each active particle i, we assign each other particle in the neighbourhood of particle i to a shell s, based on the shortest path to particle i within the Voronoi construction (see Fig. 5(a)). We then calculate the fraction of active particles within each shell, normalized by the remaining fraction of active particles in the system, i.e. Caa(s) = gaa(s)/gideal, where gaa(s) is the average fraction of active particles in shell s around an active particle, and  which in the thermodynamic limit equals α. We plot Caa(s) for various self-propulsion forces f in Fig. 5(b). For low self-propulsion fσ/kBT ≤ 30 the active particles are distributed (almost) ideally, i.e. Caa ≈ 1 for all s. At fσ/kBT = 50 we observe a large peak in Caa at small s, showing that active particles are preferentially found in close proximity: active particles cluster, in agreement with Fig. 4(c). Upon further increasing the self-propulsion we find that the peak in Caa lowers and starts to decay more slowly. This corresponds to an expansion of the defect clusters which distributes the active particles over a larger area. At high self-propulsion fσ/kBT ≥ 80 we see that the clustering becomes much less pronounced, as can also be seen from Fig. 4(e).
which in the thermodynamic limit equals α. We plot Caa(s) for various self-propulsion forces f in Fig. 5(b). For low self-propulsion fσ/kBT ≤ 30 the active particles are distributed (almost) ideally, i.e. Caa ≈ 1 for all s. At fσ/kBT = 50 we observe a large peak in Caa at small s, showing that active particles are preferentially found in close proximity: active particles cluster, in agreement with Fig. 4(c). Upon further increasing the self-propulsion we find that the peak in Caa lowers and starts to decay more slowly. This corresponds to an expansion of the defect clusters which distributes the active particles over a larger area. At high self-propulsion fσ/kBT ≥ 80 we see that the clustering becomes much less pronounced, as can also be seen from Fig. 4(e).
Intuitively, one may expect that at low self-propulsion all self-propelled particles are caged. On the other hand, if the self-propulsion is high we expect active particles to be mobile. To examine the presence or absence of this dynamical transition we calculate the fraction of mobile active particles 〈pm〉, defined as particles that have moved more than one lattice spacing Δr ≥ a within a sufficiently long time interval Δt = 2.5τ.36 In Fig. 6(a) we plot 〈pm〉 as a function of f for varying densities. While at low self-propulsions f all active particles remain caged 〈pm〉 = 0, we observe a sudden increase in the average number of mobile active particles beyond some critical f, indicating a sudden onset of cage breaking [Fig. 6(a)]. With increasing density ρ the lattice becomes more rigid, making the jump in 〈pm〉 shift to higher f as the active particles require more force in order to break from their cage.
 | ||
| Fig. 6 (a) The average fraction of mobile self-propelled particles 〈pm〉. (b) The average fraction of defects 〈pd〉. (c) State diagram indicating the regimes where active particles are caged vs. mobile. (d) In the mobile regime, ρσ2 = 0.845 and fσ/kBT = 90, we observe significant coarsening of polycrystals. Here active particles accumulate at the grain boundaries. (e) In the caged regime, ρσ2 = 0.845 and fσ/kBT = 30, the grain growth occurs very similarly to the purely passive system. Here active particles remain inside the crystal domains and no enhanced grain growth occurs. Color-coding the same as in Fig. 1. α = 0.004 in all plots. | ||
We further quantify the dynamics of self-propelled particles for various self-propulsions, we calculate the mean square displacement, see Fig. 7 for α = 0.004 at ρσ2 = 0.845. While, at very short time scales (t/τ ≲ 10−3) all mean square displacements overlap, we can see that at longer time scales the dynamics of active particles are separated into two groups. Clearly, at low self-propulsions fσ/kBT ≤ 40 particles remain (more or less) caged, while at higher self-propulsion the mean square displacement of the active particles exhibits a strong increase over time. Such an increase of the long-term mean square displacement with increasing activity is in good agreement with the data shown in Fig. 6(a).
 | ||
| Fig. 7 Mean square displacement 〈Δr2(t)〉 of self-propelled particles at ρσ2 = 0.845 with an active fraction of α = 0.004 for different strengths of self-propulsion fσ/kBT. | ||
We also observe that the increase in mobility of active particles strongly correlates with the formation of defects, as monitored by the average fraction of defects 〈pd〉 inside the crystals, shown in Fig. 6(b). The onset of defect formation (〈pd〉 > 0) coincides perfectly with the large jump observed in 〈pm〉. With increasing density ρ, the crystal becomes stiffer and defects form at higher self-propulsions. The interplay between the self-propulsion f and the density ρ, which respectively promote and restrict diffusion of active particles, is captured in a state diagram, as shown in Fig. 6(c). Here we used the average fraction of mobile active particles as a cutoff 〈pm*〉 > 0.1 to differentiate between the two regimes where active particles are caged or mobile.
This cage-breaking transition plays a crucial role in active grain growth. Specifically we only observe significant active grain growth in systems in which the active particles are mobile. Upon activation, self-propelled particles accumulate at the grain boundaries, causing many particles at the surface of the grains to rearrange, thus leading to significant grain boundary motion over time [Fig. 6(d)]. However, in systems where active particles are caged, the active particles cannot diffuse to the grain boundaries [Fig. 6(e)] and we observe no such enhancement in the grain growth; the grain growth is very similar to a purely passive system. Hence, active particles enhance grain growth only when they can easily diffuse to the grain boundaries.
Conclusions
In conclusion, we have explored the doping of two-dimensional colloidal (poly)crystals with sparse active particles. We find that active particles enhance the mobility of grain boundaries, leading to large-scale single-domain crystals containing local defect clusters rich in active particles. Further analysis on single crystals revealed an intricate relation between the dynamics of active particles and the formation, and motion of defects in two-dimensional colloidal crystals. Not only do defects play a pivotal role in determining the mobility of active particles, they also generate attractions between active particles, which eventually drive the system to “phase separate” into mostly passive hexagonal crystal domains and complex defect clusters that are rich in active particles.Our study demonstrates a novel avenue for removing grain boundaries – simply turning on and off the self-propulsion of the active particles. Hence by simply controlling the energy source for the active particles, via e.g. the availability of fuel or an external light source, active particles can be used to grow large-scale single-domain crystals.
We acknowledge funding from the Dutch Sector Plan Physics and Chemistry, and funding from a NWO-Veni grant. We thank Vasileios Prymidis, Frank Smallenburg and Sela Samin for careful reading of the manuscript.
References
- S. Gokhale, K. H. Nagamanasa, R. Ganapathy and A. Sood, Soft Matter, 2013, 9, 6634 RSC.
- Y.-H. Ye, F. LeBlanc, A. Haché and V.-V. Truong, Appl. Phys. Lett., 2001, 78, 52 CrossRef CAS.
- Z. Cheng, W. B. Russel and P. Chaikin, Nature, 1999, 401, 893 CrossRef CAS PubMed.
- A. Van Blaaderen, R. Ruel and P. Wiltzius, Nature, 1997, 385, 321 CrossRef CAS.
- K.-h. Lin, et al. , Phys. Rev. Lett., 2000, 85, 1770 CrossRef CAS PubMed.
- P. V. Braun, et al. , Adv. Mater., 2001, 13, 721 CrossRef CAS.
- M. Allard, E. H. Sargent, P. C. Lewis and E. Kumacheva, Adv. Mater., 2004, 16, 1360 CrossRef CAS.
- T. Palberg, W. Mönch, J. Schwarz and P. Leiderer, J. Chem. Phys., 1995, 102, 5082 CrossRef CAS.
- R. Amos, et al. , Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2000, 61, 2929 CrossRef CAS.
- S. Gokhale, et al. , Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 20314 CrossRef CAS PubMed.
- A. Sokolov and I. S. Aranson, Phys. Rev. Lett., 2012, 109, 248109 CrossRef PubMed.
- J. Schwarz-Linek, et al. , Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 4052 CrossRef CAS PubMed.
- W. F. Paxton, et al. , J. Am. Chem. Soc., 2004, 126, 13424 CrossRef CAS PubMed.
- R. Dreyfus, et al. , Nature, 2005, 437, 862 CrossRef CAS PubMed.
- J. R. Howse, et al. , Phys. Rev. Lett., 2007, 99, 048102 CrossRef PubMed.
- I. Theurkauff, et al. , Phys. Rev. Lett., 2012, 108, 268303 CrossRef CAS PubMed.
- I. Buttinoni, et al. , Phys. Rev. Lett., 2013, 110, 238301 CrossRef PubMed.
- J. Palacci, et al. , Science, 2013, 339, 936 CrossRef CAS PubMed.
- J. Stenhammar, et al. , Phys. Rev. Lett., 2013, 111, 145702 CrossRef PubMed.
- J. Stenhammar, D. Marenduzzo, R. J. Allen and M. E. Cates, Soft Matter, 2014, 10, 1489 RSC.
- R. Wittkowski, et al. , Nat. Commun., 2014, 5, 4351 CAS.
- J. Bialké, H. Löwen and T. Speck, Europhys. Lett., 2013, 103, 30008 CrossRef.
- G. S. Redner, M. F. Hagan and A. Baskaran, Phys. Rev. Lett., 2013, 110, 055701 CrossRef PubMed.
- T. Speck, J. Bialké, A. M. Menzel and H. Löwen, Phys. Rev. Lett., 2014, 112, 218304 CrossRef.
- Y. Fily and M. C. Marchetti, Phys. Rev. Lett., 2012, 108, 235702 CrossRef PubMed.
- Y. Fily, S. Henkes and M. C. Marchetti, Soft Matter, 2014, 10, 2132 RSC.
- V. Narayan, S. Ramaswamy and N. Menon, Science, 2007, 317, 105 CrossRef CAS PubMed.
- J. Deseigne, O. Dauchot and H. Chaté, Phys. Rev. Lett., 2010, 105, 098001 CrossRef PubMed.
- V. Schaller, et al. , Nature, 2010, 467, 73 CrossRef CAS PubMed.
- R. Ni, M. A. C. Stuart, M. Dijkstra and P. G. Bolhuis, Soft Matter, 2014, 10, 6609 RSC.
- F. Kümmel, et al. , Soft Matter, 2015, 11, 6187 RSC.
- J. Stenhammar, R. Wittkowski, D. Marenduzzo and M. E. Cates, Phys. Rev. Lett., 2015, 114, 018301 CrossRef PubMed.
- T. O. Skinner, D. G. Aarts and R. P. Dullens, Phys. Rev. Lett., 2010, 105, 168301 CrossRef PubMed.
- W. Lechner and C. Dellago, Soft Matter, 2009, 5, 2752 RSC.
- W. Lechner, et al. , Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2013, 88, 060402 CrossRef PubMed.
- Note that we checked that our results are robust to variations in both the displacement threshold as well as the time-interval.
| This journal is © The Royal Society of Chemistry 2016 |



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 446 and the density is
446 and the density is