Hydrogen bonding strength of diblock copolymers affects the self-assembled structures with octa-functionalized phenol POSS nanoparticles
Abstract
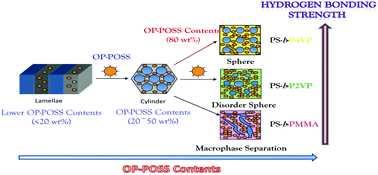
In this study, the influence of the functional groups by the diblock copolymers of poly(styrene-b-4-vinylpyridine) (PS-b-P4VP), poly(styrene-b-2-vinylpyridine) (PS-b-P2VP), and poly(styrene-b-methyl methacrylate) (PS-b-PMMA) on their blends with octa-functionalized phenol polyhedral oligomeric silsesquioxane (OP-POSS) nanoparticles (NPs) was investigated. The relative hydrogen bonding strengths in these blends follow the order PS-b-P4VP/OP-POSS > PS-b-P2VP/OP-POSS > PS-b-PMMA/OP-POSS based on the Kwei equation from differential scanning calorimetry (DSC) and Fourier transform infrared spectroscopic analyses. Small-angle X-ray scattering and transmission electron microscopic analyses show that the morphologies of the self-assembly structures are strongly dependent on the hydrogen bonding strength at relatively higher OP-POSS content. The PS-b-P4VP/OP-POSS hybrid complex system with the strongest hydrogen bonds shows the order–order transition from lamellae to cylinders and finally to body-centered cubic spheres upon increasing OP-POSS content. However, PS-b-P2VP/OP-POSS and PS-b-PMMA/OP-POSS hybrid complex systems, having relatively weaker hydrogen bonds, transformed from lamellae to cylinder structures at lower OP-POSS content (<50 wt%), but formed disordered structures at relatively high OP-POSS contents (>50 wt%).

 Please wait while we load your content...
Please wait while we load your content...