Counter-ion distribution around flexible polyelectrolytes having different molecular architecture
Alexandros
Chremos
* and
Jack F.
Douglas
*
Materials Science and Engineering Division, National Institute of Standards and Technology, Gaithersburg, MD 20899, USA. E-mail: alexandros.chremos@nist.gov; jack.douglas@nist.gov
First published on 2nd February 2016
Abstract
We explore the monovalent counter-ion distribution around flexible highly-charged polyelectrolytes with different molecular architectures (linear chains, stars, and unknotted and trefoil rings) using molecular dynamics simulations that include an explicit solvent that interacts with the polyelectrolyte. In particular, we find that the molecular topology influences the fraction of counter-ions transiently associating with the polyelectrolyte on a scale of the order of the chain segments, forming a “condensed” counter-ion interfacial layer. As with the hydrogen bonding of water to proteins and other polymers, the persistence time of these interfacial “bound” counter-ions is relatively short, O(1 ps), and we characterize the fluctuations in the number of the counter-ions populating the interfacial layer. We also find that the counter-ions are distributed in a non-uniform fashion on the polyelectrolyte backbone, forming dynamical clusters whose form and average size is sensitive to molecular architecture. In addition, we find that the residual bound counter-ions, not located in either the interfacial layer or the bulk solution, form a diffuse ionic cloud around the polyelectrolyte due to the uncompensated polyelectrolyte charge along the backbone. Generally charge valence strongly influences the extent of the diffuse counter-ion cloud, but in the case of monovalent counter-ions, we find that the size of the diffuse counter-ion cloud nearly coincides with the polyelectrolyte radius of gyration, independent of molecular topology.
1 Introduction
Polyelectrolytes form a diverse class of polymers that includes biological molecules such as DNA, RNA, proteins, and synthetic polymers such as sulfonated polystyrene and polyacrylic acid. These polymers are widely used as rheology modifiers, adsorbent materials, coatings, biomedical implant materials, encapsulating materials for pharmaceutical drug delivery systems1–3 and, of course, they are an essential component of living systems. A microscopic understanding is needed of how the counter-ion distribution influences the polyelectrolyte configurational properties,4 which are of fundamental importance in the inherent tendency of polyelectrolytes to undergo supermolecular assembly,5,6 their non-trivial phase behavior,7,8 as well as for understanding the unique rheological and dielectric properties of these materials.8–10As with many ionizable materials, polyelectrolytes release their counter-ions into polar solvents in which they are dissolved,11,12 but the extent of this counter-ion delocalization is modulated by molecular structural parameters such as charge density, charge valence, chain length, and molecular topology to name a few relevant molecular variables. It is generally appreciated that this ionization process leads to a charged chain backbone in aqueous solution and results in long-range repulsive Coulomb interactions between the polymer segments that cause the polymer to swell. The strength of this interaction must be greatly influenced by counter-ions that remain in the general proximity of the polyelectrolyte, but which are not ionically bound as in a crystal. It is these weakly bound counter-ions and their effect on chain conformation that make the modeling of polyelectrolytes challenging.13–16 Given the evident complexity of the coupling between polymer conformation and the counter-ion distribution, analytic theories of polyelectrolytes have emphasized highly idealized models aimed at describing some essential aspects of the localization of counter-ions near the polyelectrolyte and the consequences of this association on the thermodynamics of polyelectrolyte solutions.
The classical theoretical approach to counter-ion localization in highly charged polyelectrolytes was introduced by Manning.17,18 According to this model, when the electrostatic interactions dominate over thermal fluctuations then the fully-ionized state of the polyelectrolyte becomes unstable and counter-ions “condense” along the chain backbone, giving rise to a characteristic counter-ion “localization length” on the order of the Bjerrum length, lB = e2/(εrkBT) (e is the elementary charge, εr is the solvent dielectric constant, T is the temperature, and kB is Boltzmann's constant). Manning theory17,18 is a rather idealized model that assumes that highly charged polyelectrolytes are infinite straight threads having vanishing diameter and a uniform charge density. Counter-ion localization does not theoretically arise in the model when the polyelectrolytes have a finite chain length and this model does not consider how changes in conformation induced by the counter-ions and thermal fluctuations of the chain backbone alter the physical nature of the counter-ion localization to the polyelectrolyte. Simulation studies7,19–27 of flexible polyelectrolytes having finite length indicate significant deviations from Manning theory, and provide evidence for the importance of the coupling of the counter-ions with polymer conformation.
The development of more realistic analytic models of polyelectrolytes have followed two approaches, namely an extension of the Manning model based on the mean-field Poisson–Boltzmann theory28–32 and through the introduction of continuous flexible chain model33,34 that address the influence of counter-ions on chain conformation. A valiant first attempt to better describe this coupling was made by Muthukumar33 where counter-ion condensation on the surface of a flexible polyelectrolyte chain was modeled by an association mechanism affecting the polyelectrolyte self-interaction and, thus chain conformation. In models of the first type, the polyelectrolyte is still assumed to have a rod-like conformation so that the coupling between the counter-ion distribution and the chain conformation is simply ignored. In the second type of model, the condensed ions renormalize the charge density and the dissociated counter-ions are modeled based on a simple Debye–Hückel approximation. Shklovskii35 has also made an interesting contribution to this field by showing that highly charged spherical particles can exhibit long range attractions and conductive properties that are associated with a diffuse counter-ion cloud around the particle. The influence of the diffuse counter-ion cloud on polyelectrolyte association is also emphasized in the controversial model of colloidal interactions by Sogami and Ise.36 Similar arguments have been made by Kirkwood and coworkers37 regarding the physical implications of a “polarizable” cloud of counter-ions around polyelectrolytes (modeled as charged spheres). We can then expect a diffuse counter-ion cloud to have significant implications for polyelectrolyte association and we take a first step in addressing this fundamental problem by quantifying the nature of the diffuse counter-ion cloud.
The present work directly address the coupling between the molecular conformation and the counter-ion distribution in highly charged polyelectrolytes in an explicit solvent by molecular dynamics (MD) simulations. We choose not to follow the historical pattern of previous polyelectrolyte conformation studies, in which polymer and salt charge concentrations are altered. Instead, we consider different molecular topologies (linear chains, stars, and unknotted and trefoil rings; see Fig. 1), since topology (i.e., the connectivity between the polyelectrolyte segments) influences the average molecular shape and segmental density even in the absence of charges; chain topology should then affect the coupling between chain conformation and the counter-ion distribution. Indeed, it is well known for neutral polymers that molecular topology plays an important role in the viscoelesticity of these material.38–41 Variation of the molecular topology of charged polymer is also used in improving the efficiency of water-soluble polymer drug carriers for the treatment of cancer and in the development of numerous nanomedicine therapies.42,43 Investigations on the impact of the molecular topology of polyelectrolytes are limited in comparison to linear polyelectrolytes, but there are some notable studies on star polyelectrolytes44–48 with an implicit solvent and rings with knots.49 By using an explicit solvent, we can account for the short-ranged solvent interactions needed to address the dynamical consequences of counter-ion and solvent association with the polyelectrolyte.
We find that polyelectrolytes tend to form semi-flexible polymers whose conformation is highly sensitive to molecular topology. In this more realistic model of polyelectrolytes, counter-ions localize for all molecular models both on the scale of lB as in the idealized Manning theory, but also on the scale of the chain as a whole, i.e., radius of gyration, Rg. This two scale localization has a fundamental significance for understanding intermolecular interactions in polyelectrolyte solutions.
This paper is organized as follows. Section 2 contains details of the model and simulation methods. Results of the characterization of the counter-ion distribution in the proximity to polyelectrolytes of different molecular architecture are presented in Section 3. Section 4 concludes the paper.
2 Model and methodology
We employ molecular dynamics (MD) simulations based on a bead-spring model of Lennard-Jones (LJ) segments bound by stiff harmonic bonds suspended in explicit LJ solvent particles, some of which are charged to represent counter-ions and ions from added salt. The particular model of polyelectrolytes that we employ was originally introduced by Yethiraj and coworkers,50,51 and subsequently was investigated by Koplik et al.25 This model incorporates minimal aspects of real polyelectrolyte solution and allows for equilibrated molecular dynamics with an explicit solvent. The added complexity and the cost in computational time required by inclusion of an explicit solvent is motivated by an important series of papers by Yethiraj and coworkers50,51 indicating that the solvent has a significant impact on the polyelectrolyte structure and dynamics, and, of course, one cannot reasonably model the competitive binding of the solvent and counter-ions to the polyelectrolyte backbone, an essential feature of these molecules, without including the solvent explicitly. Competitive binding of the molecular spaces to macromolecules is known to greatly alter the phase behavior of polymer solutions,52,53 leading to often counter-intuitive phase behavior. Even greater complexity can be expected when associated species have long range interactions.All macro-ion segments, dissolved ions, and solvent particles are assigned the same mass m, size σ, strength of interaction ε, and all dissolved ions are monovalent. We set ε and σ as the units of energy and length and the cutoff distance for LJ interaction potential is rc = 2.5σ. Polyelectrolyte molecular mass ranges from Mw = fM + 1 = 11 to 161 segments, where M corresponds to number of segments per arm and f represent the number of arms; f = 2 for all molecular architectures except for stars. All polyelectrolytes carry a total charge −Zpe and Zp/Mw = 1, which is distributed uniformly along the molecular structure. The bonds between polymer segments are connected via a stiff harmonic spring, VH(r) = k(r − l0)2, where l0 = σ is the equilibrium length of the spring, and k = 1000ε/σ2 is the spring constant. To model finite size rod-like polymers, we use the same model as with flexible chains, but a bending potential is used, Ubend(θ) = kbend(θ − θ0)2; where θ0 = 180° and kbend = 1000 ε/rad2. All charged particles interact via the Coulomb potential, and the particle–particle particle-mesh method is used. The operating conditions are typical of the LJ liquid state: density ρσ3 = 0.8 and reduced temperature kBT/ε = 1, the latter maintained by a Nosé–Hoover thermostat.
The system is composed of a total of 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 solvent particles in a periodic cube of side L. The system includes N− co-ions of charge −e and N+ = N− + Zp counter-ions of charge +e so that the system of interest has neutral total charge. Aside from the Bjerrum length that specifies the strength of the Coulomb interaction, the other key length parameter for an ionic solution is the Debye screening length: λD = [4πlB(ρ+ + ρ−)]−1/2, where ρ± = N±/L3 are the ion densities. Simulations are conducted for lB/σ = 1.85 and N+ = 300, which results in λD/σ ≈ 2.5. MD time unit is τ = σ(m/ε)1/2. Typical simulations equilibrate for 2000τ, and data is accumulated over a 7500τ interval.
000 solvent particles in a periodic cube of side L. The system includes N− co-ions of charge −e and N+ = N− + Zp counter-ions of charge +e so that the system of interest has neutral total charge. Aside from the Bjerrum length that specifies the strength of the Coulomb interaction, the other key length parameter for an ionic solution is the Debye screening length: λD = [4πlB(ρ+ + ρ−)]−1/2, where ρ± = N±/L3 are the ion densities. Simulations are conducted for lB/σ = 1.85 and N+ = 300, which results in λD/σ ≈ 2.5. MD time unit is τ = σ(m/ε)1/2. Typical simulations equilibrate for 2000τ, and data is accumulated over a 7500τ interval.
3 Results and discussion
We start our discussion of the counter-ion distribution in our model polyelectrolyte with the observation that the fraction of the counter-ions located in close proximity to the polyelectrolyte forms an interfacial region having an enriched average concentration of counter-ions. This can be qualitatively understood in terms of Manning model of charge condensation,17 where the counter-ions are inferred to “condense” along the chain backbone to screen the bare charge for a high charge polyelectrolyte. Nevertheless, counter-ions found in this interfacial region only partially screen the bare charge of the polyelectrolyte due to the Coulombic repulsion between the counter-ions, leaving the remaining counter-ions at larger distances from the polyelectrolyte. In other words, there are two zones of counter-ion enrichment around the polyelectrolyte. While it is commonly assumed that the interfacial layer has a scale on the order of lB, it has previously been observed that there is significant variation in the amount charge at shorter and larger distances than lB.25 This two-zone model just described is a bit ambiguous since there is no sharp boundary between the zones that would allow the quantification of the respective scales of counter-ion localization. For the purposes of our investigation, we set the interfacial layer based on an arbitrary distance criterion in which any counter-ion that is located at shorter distances than 1.1σ are taken to be part of the interfacial region. This particular value, i.e., 1.1σ, is chosen in order to discriminate between the counter-ions that are in contact with the polyelectrolyte from the remaining counter-ions.Now that we have defined the interfacial region for our model based on a precise geometric picture, we calculate the average number of interfacial counter-ions, nint, for the different molecular topologies (linear chains, rings, trefoil knots, and stars). We note that our discussion is an expansion of a preliminary study of this same topic,54 where we found that the interfacial counter-ions exhibit a rich spectrum of behaviors for the different molecular topologies. As seen in Fig. 2a, nint is influenced both by Mw and molecular topology. This is not surprising given that polyelectrolytes under investigation have a flexible backbone, while in the Manning model of charge condensation polyelectrolytes are assumed to be slender needles. Overall, the deviation from the linear chains is observed as the molecular complexity increases as in the case of stars and trefoil knots. In our previous investigation,54 we found that if instead nint is defined as the counter-ions located at shorter distances than lB (rather than 1.1σ) then a qualitative agreement is found between Manning predictions about the fraction of condensed counter-ions and our results for nint.
We digress to explain some dynamical aspects of the counter-ion enrichment. The value of nint fluctuates over time, reflecting a dynamic binding and unbinding process where the residence time of the counter-ion is rather short, O(1 ps), so that the interfacial counter-ions are exchanging with the counter-ions in the solution at a reasonably high rate.13,14,55 The solvent also interacts with the polymer with a short range interaction and likewise the interfacial solvent continuously exchanges with the solvent molecules in solution on a comparable timescale to interfacial counter-ion exchange process. Despite the rapid exchange process of both the competing counter-ion and the solvent exchange processes, there is nonetheless a time-averaged enrichment of counter-ions near the polyelectrolyte defining a “bound” layer of counter-ions; this is an equilibrium self-assembly phenomenon.25 Below, we discuss the spatial organization of the counter-ions in the interfacial layer, which is normally far from uniform even in our coarse-grained model.
The magnitude of the fluctuations for nint can be quantified by the standard deviation, Σ(nint). Linear chains and rings exhibit similar values for nint and for Σ(nint) signifying that the molecular complexity of the rings is not sufficient to distinguish them from linear chains, as seen in Fig. 2a and c. On the other hand, we see that trefoil knots exhibit fluctuations of a similar magnitude, but have quite different values of nint. Stars exhibit both nint and fluctuations in this property that are conspicuously distinct from linear chains and ring polyelectrolytes. Based on these observations, we conclude that chain topology strongly influences the population of counter-ions at the interfacial layers, as well as fluctuations in their numbers.
In our previous work56 exploring the effect of multivalent counter-ions on linear chains, we argued that the observed conformational changes are a result of the chain coiling around the polyelectrolytes, Fig. 2b. A coiling of the chains is suggested by the increase in the number of contacts the interfacial counter-ions have with the polyelectrolyte, ncont. While we find similar features as for nint, the contact number ncont approaches a similar level, ncont/Mw ≈ 0.37 for all molecular topologies simulated. For rings, ncont/Mw exhibits little Mw variation, while for linear chains ncont/Mw decreases with Mw for small Mw. On the other hand, for trefoil knots and stars there is a substantial increase in ncont/Mw for small Mw, signifying that these molecular topologies adopt molecular conformations that wrap the interfacial counter-ions. This counter-ion enrichment in flexible polyelectrolytes can be understood by excess counter-ions found in kinks and near the branched points of charged lines.57 This trend is also reflected in the size of the fluctuations in ncont, as indicated in Fig. 2d. Based on the saturation behavior of ncont, we anticipate that ncont will be an important factor in determining the energetics and equilibrium properties, but to our knowledge this quantity has not been considered in polyelectrolyte modeling before.
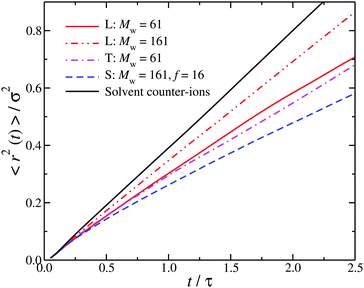
The role of molecular topology in wrapping around interfacial counter-ions suggests that this effect should also be reflected in the interfacial counter-ion dynamics. The fast dynamics probed by neutron scattering in the picosecond time scale (t/τ < 2.5, where τ ≈ 1 ps) can be utilized to define a Debye–Waller factor for the interfacial counter-ions. While a time scale on the order of a 1 ps sounds relatively short to have an impact on the macroscopic dynamics, we note that there are studies that argue that while the water dynamics in the hydration layer around proteins and other polymers is of a few picoseconds, it is sufficiently long to affect the polyelectrolyte hydrodynamics.58–61 Accordingly, we calculate the mean-square displacement, 〈r2〉, for the interfacial counter-ions. To ensure that the 〈r2〉 is not correlated with the particular polyelectrolyte conformation and that the counter-ions in the interfacial layer have not diffused away from the polyelectrolyte, we have averaged our results from ten different uncorrelated time origins. From Fig. 3, it is clear that the association between counter-ions and chain backbone influences the interfacial dynamics, with higher branched polyelectrolytes leading to a significant reduction in mobility (as measured by 〈r2〉). In hindsight, this is not surprising. Fig. 1e shows a star polyelectrolyte with f = 16, and it clear that a significant fraction of counter-ions are located near the star core.
Based on the above discussion, we now have a more detailed picture on how the interfacial counter-ions associate with polyelectrolytes having different molecular topologies, and we now shift our focus towards to the counter-ions that are in the proximity of the polyelectrolyte, but are not part of the “bound” interfacial layer, as described above. To better quantify the distribution of counter-ions around the polyelectrolyte, we calculate the average net charge, q(r), as function of distance from the polyelectrolyte segments, which is simply the sum of the counter-ion distribution q+(r) and the co-ion distribution q−(r). For comparison, we also calculate the distribution of counter-ions belonging to the interfacial bound layer alone, qb. Evidently from Fig. 4, q(r) represents both the counter-ions at the interfacial layer and the counter-ion cloud that is loosely associated with the polyelectrolyte. From Fig. 4, it is also clear, that qb follows the distribution of the polyelectrolyte segments but always corresponds to a smaller amount of charge; this is expected as the counter-ions at the interfacial layer only partially screen the polyelectrolyte bare charge. On the other hand, there are significant deviations between q(r) and qb starting at distances on the order of lB, signifying that qb is insufficient metric for accounting the whole impact of counter-ions near the polyelectrolyte. To understand why q(r) is a better metric for capturing the distribution of the counter-ions, we compare the relevant distributions of each type of ion. The location of the boundary, Rb, between the counter-ion cloud around the polyelectrolyte and the bulk solvent can be identified at q(Rb) → 0, which means that q+ = q−. Thus, we have q(r) ≈ 0 for distances r > Rb, while on the other hand, there is an excess counter-ion charge and a depletion of co-ion charges for r < Rb, corresponding to the diffuse counter-ions around the polyelectrolyte. The latter regime apparently embodies what has been described in the literature as the “diffuse counter-ion cloud”, as discussed in the introduction. We refer to q(r) as the counter-ion distribution of the diffuse ionic cloud (including the counter-ions in the interfacial layer) and focus on characterizing the spatial extent of this distribution.
At this point, one might naturally expect q(r) to track the segmental distribution since molecular conformations are coupled to the counter-ion distribution. Fig. 4 displays both the segmental and counter-ion distributions, which include the interfacial and diffusely associated counter-ions. It is clear that at short distances, near the polyelectrolyte backbone segments, there is more charge from neighboring segments than from counter-ions, q(r) < qp(r). This means that at short distances there is a higher population of polyelectrolyte segments than counter-ions, and this imbalance results in an effective repulsion between the segments, leading to a swelling of the polyelectrolyte. Nevertheless, at larger distances there is a crossover for q(r) > qp(r). For example, a trefoil knot having Mw = 61, as seen in Fig. 4, the crossover occurs at r > 6.5σ. This means that at distances r > 6.5σ, there is a higher probability of finding a monovalent counter-ion than a polyelectrolyte segment. This is also reflected on the slower decay of the tail of the counter-ion distribution than the segmental distribution. This is significant because it means that q(r) includes a large fraction of counter-ions that are not in the interfacial layer. Thus, the counter-ion distribution has a structure that is distinct from the segmental distribution, an effect warranting quantification.
To determine the spatial extent of the diffuse counter-ion cloud, we compute the average ionic charge at a distance r from any macro-ion segment, q(r), as can be seen in Fig. 5. In close proximity to the macro-ion, there is an oscillatory behavior in q(r), reflecting liquid-like correlations in the counter-ions around the macro-ion, and these correlations diminish beyond a scale of 2lB. This effect is more pronounced in finite length rod polyelectrolytes due to high spatial regularity of the macro-ion charges (note that we do not observe waves of oscillatory charge for the conditions of our investigation). For relative small Mw < 41, q(r) decays to zero at larger distances. For molecular architectures other than rods and for Mw > 41, q(r) exhibits a substantial peak occurring at a scale much larger than λD. This strongly suggests the existence of another length scale describing the distribution of the counter-ion cloud around these polyelectrolytes.
Numerous recent experimental and computational studies of polyelectrolytes have definitively established the importance of the diffusely bound counter-ions in the understanding the properties of polyelectrolyte solutions,13–16,24 but we are not aware of any previous computational work quantifying the scale of this “cloud”. For example, we seek to understand how the spatial extent of this diffuse counter-ion cloud varies with the polyelectrolyte mass or molecular architecture. The lack of quantification of this basic polyelectrolyte property is surprising to us given the importance of this phenomenon for understanding polyelectrolyte properties and intermolecular interactions.13–16,62–65
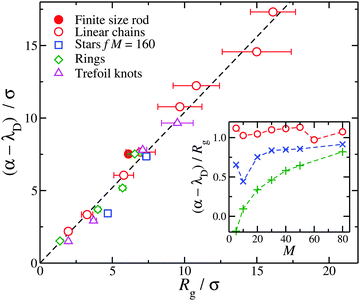
We take a cue from previous work66 based on PB calculations for rod polyelectrolytes suggesting that a localization scale can be defined from the inflection point in the net ionic charge  at a distance r from macro-ion segments. This scale is certainly larger than lB and λD, so we consider this quantity for our flexible polymers to see if we can define a rational metric for counter-ion localization based on Q(r); see inset of Fig. 6. It is evident that Q(r) increases and then saturates at long distances at Q(r)/Zp ≈ 1 for all molecular architectures examined. We observe that macro-ions with different Mw (since Zp = λMw) have different levels of charge saturation. We also find that the molecular complexity regulates the rate of saturation in Q(r). For all the cases examined (rings, trefoil knots, and stars) Q(r), which saturates at shorter distances in comparison to linear chains having the same M (see inset of Fig. 6).
at a distance r from macro-ion segments. This scale is certainly larger than lB and λD, so we consider this quantity for our flexible polymers to see if we can define a rational metric for counter-ion localization based on Q(r); see inset of Fig. 6. It is evident that Q(r) increases and then saturates at long distances at Q(r)/Zp ≈ 1 for all molecular architectures examined. We observe that macro-ions with different Mw (since Zp = λMw) have different levels of charge saturation. We also find that the molecular complexity regulates the rate of saturation in Q(r). For all the cases examined (rings, trefoil knots, and stars) Q(r), which saturates at shorter distances in comparison to linear chains having the same M (see inset of Fig. 6).
 | ||
| Fig. 6 Normalized spatial distribution of the net ionic charge around a macro-ion of various molecular architectures. The notation is the same as in Fig. 2. Inset: Net ionic distribution without being normalized. | ||
Our findings up to this point regarding the nature of the counter-ion distribution can be rationalized within existing polyelectrolyte theories, however, we find that all our Q(r) curves collapse onto a single master curve with the following functional form:
Q(r) = βZp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tanh2[(r − μ)/α], tanh2[(r − μ)/α], | (1) |
| Topology | f | M | Z p | α | β | μ |
|---|---|---|---|---|---|---|
| L | 2 | 5 | 11 | 4.61 | 0.92 | 0.00 |
| L | 2 | 10 | 21 | 5.79 | 1.00 | 0.00 |
| L | 2 | 20 | 41 | 8.54 | 1.00 | 0.00 |
| L | 2 | 30 | 61 | 10.18 | 1.00 | 0.00 |
| L | 2 | 40 | 81 | 13.36 | 1.00 | 0.00 |
| L | 2 | 50 | 101 | 14.86 | 1.00 | 0.00 |
| L | 2 | 60 | 121 | 17.25 | 1.00 | 0.00 |
| L/S | 2 | 80 | 161 | 20.12 | 1.00 | 0.00 |
| S | 4 | 40 | 161 | 22.56 | 1.54 | −0.57 |
| S | 8 | 20 | 161 | 10.16 | 1.04 | 1.33 |
| S | 16 | 10 | 161 | 6.23 | 1.00 | 0.94 |
| R | 2 | 5 | 11 | 3.95 | 0.79 | −0.27 |
| R | 2 | 20 | 41 | 6.19 | 1.00 | 0.56 |
| R | 2 | 30 | 61 | 7.71 | 1.04 | 0.89 |
| R | 2 | 40 | 81 | 10.1 | 1.07 | 0.66 |
| R | 2 | 60 | 121 | 17.6 | 1.26 | −0.18 |
| R | 2 | 80 | 161 | 43.11 | 4.01 | −0.69 |
| T | 2 | 15 | 31 | 3.97 | 0.97 | −0.01 |
| T | 2 | 30 | 61 | 5.46 | 0.99 | 0.43 |
| T | 2 | 51 | 103 | 10.43 | 1.06 | 0.08 |
| T | 2 | 80 | 161 | 12.46 | 1.09 | 0.74 |
| F | 2 | 10 | 21 | 10.09 | 1.02 | −0.56 |
| F | 2 | 20 | 41 | 21.02 | 1.32 | −2.22 |
Why should we be concerned about the diffuse counter-ion cloud? Apart from the large effect this layer has on the dielectric properties of polyelectrolyte solutions,67 we may expect this loosely associated cloud of counter-ions to make polyelectrolytes highly polarizable. Kirkwood and coworkers37 have predicted that the presence of the “polarizable” cloud of counter-ions around polyelectrolyte chains (proteins modeled as spheres) can lead to long range attractions interactions arising from the associated fluctuations of the potential field derived from fluctuations in the positions of the counter-ions around the polyelectrolyte. Recent computational studies have shown that polarizable dielectric particles have an inherent tendency to cluster,68 so we believe that the presence of a diffuse counter-ion cloud provides a natural physical mechanism for understanding the self-assembly of polyelectrolytes into dynamic clusters.36,69–72 In the future, we plan to quantify the nature of the interchain interactions in our system to check this hypothesis.
The presence of the polyelectrolyte in the solution has a large impact not only on the average distribution of the counter-ions, but induces the self-assembly of the counter-ions within the interfacial region of the polyelectrolyte, an effect relevant to the understanding the charge fluctuation phenomenon just described. The local distribution of these associated counter-ions in the interfacial layer reflects a balance between the Coulomb attractive or repulsive interactions, and between the ions and the polyelectrolyte segments. While the formation of dynamical counter-ion clusters on the backbone of the polyelectrolyte chains have been noted before in this model,25 here we expand upon these findings by investigating the formation of counter-ion clusters for polyelectrolytes of different molecular topology. To calculate the size of a counter-ion cluster, nc, we use a distance criterion, where a counter-ion belongs to a cluster if it is located 1.2σ from a counter-ion belonging to the cluster. Moreover, we label the probability of finding a counter-ion cluster of size nc as Pc(nc), and the resulting counter-ion cluster probabilities for different molecular topologies are presented in Fig. 8. We again emphasize that these counter-ion clusters are transient, meaning that they continuously form, break apart, and then reform; no clustering is observed away from the polyelectrolyte backbone or in the absence of the polyelectrolyte under the thermodynamic conditions, i.e., salt concentration and temperature that we investigate. We find little variation in Pc(nc) for polyelectrolyte chains having different molecular mass. Additionally, for polyelectrolyte chains and rings, the size of counter-ion clusters are relative small, e.g., for Pc(nc) = 10−2, we find nc ≈ 5. The functional form of Pc appears to be nearly exponential, which is consistent with a linear chain equilibrium polymerization of the counter-ions on the polyelectrolyte backbone.73 In other words, the counter-ion clusters dynamically form chains on the polyelectrolyte backbone so that the spatial organization of these clusters loosely reflects the geometry of the polyelectrolyte that they “dress”, see Fig. 1b–e. We anticipate that the timescale of these counter-ion clusters will be longer than the exchange time, discussed above, and that these clusters will influence the charge fluctuations of the polyelectrolyte as a whole. This is an additional topic for future investigation, and for the present we focus on the geometrical nature of these curious self-assembled structures.
For trefoil knot and highly branched star polyelectrolytes, however, we see a new feature emerging in the interfacial counter-ion clustering. Large clusters form with respect to linear polyelectrolyte chains, having a mass cluster size several times larger. In particular, for stars having Mw = 161 and f = 16, we find nc ≈ 45 for Pc(nc) = 10−2; this cluster size is nine times larger than in the case of the linear polyelectrolytes discussed above. This is a highly counter-intuitive result given that counter-ions strongly repel each other. This suggests that the high charge density created by the star molecular topology, in conjunction with the more complex chain topological structure, triggers the formation of much larger counter-ion clusters that are themselves branched in nature. Consistent with this picture, the probability distribution, provided that nc is sufficiently large, nc > 5, follows a power law with cluster size, Pc(nc) ∝ nc−1.9, for large polyelectrolyte stars and trefoil knots. This behavior is reminiscent of the equilibrium polymerization of branched polymers.74
Finally, we explore the radius of gyration of the counter-ion clusters, Rg,c, in the interfacial layer. We observe that once the cluster size, nc, is sufficiently large, i.e., nc > 5, then two distinct behaviors emerge, Fig. 9. For 4 < nc < 20, the size of the counter-ion clusters scales as Rg,c ∝ nc1/2 for all molecular architectures. This is consistent that the counter-ions forming linear chain-like clusters on the linear on the backbone of the polyelectrolyte with the statistics of linear random coils, as indicated above. We thus have further evidence that dynamic counter-ion clusters are formed along the polyelectrolyte backbone, affected by the geometrical structure of the polyelectrolyte. Unfortunately, the mass scaling of the cluster radius does not allow us to distinguish between random walk counter-ion cluster conformation and randomly branched clusters (“lattice animals”), which exhibit the same scaling behavior with cluster mass as ideal linear chain coils in three dimensions,75i.e., Rg ∼ nc1/2. Stars having f = 16 and M = 10 have a high enough charge density and branched complexity for the formation of even larger counter-ion clusters nc > 20, and in these branched polyelectrolytes we see another regime emerge. In particular, highly branched counter-ion clusters are then formed, and Rg,c scales as nc to an apparent power that is near ν ≈ 0.2, suggesting that the counter-ion clusters have fully saturated the capacity of the polyelectrolyte to accommodate them on their surface, an effect similar to what happens in the mass scaling in gels (formed by particles) above the percolation threshold when the box size is small. In the present instance, the polyelectrolyte chain is the “box”. We note that a similar mass scaling crossover is physically observed in the radius of gyration of dendrimer molecules having a high generation number where excluded volume interactions inhibit further cluster growth.76 The organization of the counter-ions into self-assembled structures on the polyelectrolyte backbone is evidently a physically rich phenomenon with many implications for the conformation of polyelectrolytes and the interparticle responsible for the supramolecular assembly of the polyelectrolytes. An understanding of these interactions and assembly processes is clearly important in the design of novel functional materials using polyelectrolytes.
4 Conclusions
In summary, we quantify the distribution of monovalent counter-ions for molecular architectures (linear chains, stars, and unknotted and trefoil rings) using molecular dynamics simulations. The fraction of interfacially “bound” counter-ions, defined by an enrichment on a scale on the order of the size of the polyelectrolyte segments, is influenced by the molecular architecture. An effect not anticipated by Manning theory. We find that for molecular architectures having a higher molecular complexity than linear chains, the polyelectrolyte tends to wrap around these counter-ions, resulting in a modified counter-ion fast dynamics, as well as a different degree of screening of the bare charge of the polyelectrolyte. On the scale of the polymer radius of gyration, there is an additional diffuse counter-ion cloud that forms around the polyelectrolyte chain due to the residual unscreened polyelectrolyte charge. We demonstrate that this diffuse counter-ion distribution is independent of molecular topology for monovalent counter-ions, i.e., the extent of the counter-ion distribution scales comparable to the radius of gyration of polyelectrolyte. However, this effect is unique to monovalent counter-ions since for higher valent counter-ions the diffuse counter-ion cloud becomes more contracted and even partially collapses for trivalent counter-ions.56 Finally, we characterize the self-assembly of counter-ions into dynamic clusters in the interfacial region around polyelectrolytes having different molecular topologies. We find that the counter-ions organize into dynamic polymer-like structures near the polyelectrolyte backbone and that the topology of these clusters largely follow the topology of the polyelectrolyte backbone. These counter-ion clusters are the direct physical expression of interfacial charge fluctuations and must have relevance for understanding polyelectrolyte intermolecular interactions. Experimental synthesis and characterization of polyelectrolyte systems can be used to validate the predictions of the present work. In the future, we plan to investigate how the counter-ion structure in this family of polyelectrolyte models influence the interaction between these molecules.Acknowledgements
Financial support was provided by the NIST Fellow Postdoctoral Program. Official contribution of the U.S. National Institute of Standards and Technology – not subject to copyright in the United States.References
- P. G. de Gennes, P. Pincus, F. Brochard and R. M. Velasco, J. Phys., 1976, 37, 1461–1476 CAS.
- A. V. Dobrynin and M. Rubinstein, Prog. Polym. Sci., 2005, 30, 1049–1118 CrossRef CAS.
- A. Yethiraj, J. Phys. Chem. B, 2009, 113, 1539–1551 CrossRef CAS PubMed.
- V. M. Prabhu, Curr. Opin. Colloid Interface Sci., 2005, 10, 2 CrossRef CAS.
- C. F. J. Faul and M. Anttonietti, Adv. Mater., 2003, 15, 673–683 CrossRef CAS.
- S. Srebnik and J. F. Douglas, Soft Matter, 2011, 7, 6897–6905 RSC.
- R. G. Winkler, M. Gold and P. Reineker, Phys. Rev. Lett., 1997, 80, 3731–3734 CrossRef.
- Polyelectrolytes Thermodynamics and Rheology, ed. P. M. Visakh, O. Bayraktar and G. Pico, Springer, 2014 Search PubMed.
- A. Y. Kasumov, D. V. Klinov, P.-E. Roche, S. Gueron and H. Bouchiat, Appl. Phys. Lett., 2004, 84, 1007–1009 CrossRef CAS.
- S. Duhr and D. Braun, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 19678–19682 CrossRef CAS PubMed.
- F. Oosawa, Polyeletrolytes, Marcel Dekker, New York, 1971 Search PubMed.
- J.-L. Barat and J.-F. Joanny, Adv. Chem. Phys., 1996, 94, 1 CrossRef.
- D. Hinderberger, G. Jeschke and H. W. Spiess, Macromolecules, 2002, 355, 9698 CrossRef.
- D. Hinderberger, H. W. Spiess and G. Jeschke, J. Phys. Chem. B, 2004, 108, 3698 CrossRef CAS.
- T. E. Angelini, L. K. Sanders and R. H. Colby, J. Phys.: Condens. Matter, 2005, 17, S1123 CrossRef CAS.
- Y. Katsumoto, S. Omori, D. Yamamoto, A. Yasuda and K. Asami, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2007, 75, 011911 CrossRef PubMed.
- G. S. Manning, J. Chem. Phys., 1969, 51, 924–933 CrossRef CAS.
- G. S. Manning, J. Chem. Phys., 1969, 51, 3249 CrossRef CAS.
- M. J. Stevens and K. Kremer, J. Chem. Phys., 1995, 103, 1669–1690 CrossRef CAS.
- J. C. Chu and C. H. Mak, J. Chem. Phys., 1999, 110, 2669–2679 CrossRef CAS.
- H. J. Limbach and C. Holm, J. Chem. Phys., 2001, 114, 9674–9682 CrossRef CAS.
- S. Liu and M. Muthukumar, J. Chem. Phys., 2002, 116, 9975 CrossRef CAS.
- M. Ullner and C. E. Woodward, Macromolecules, 2002, 35, 1437–1445 CrossRef CAS.
- M. Muthukumar, J. Chem. Phys., 2002, 116, 9975 CrossRef.
- T. S. Lo, B. Khusid and J. Koplik, Phys. Rev. Lett., 2008, 100, 128301 CrossRef PubMed.
- J.-M. Y. Carrillo and A. V. Dobrynin, Macromolecules, 2011, 44, 5798–5816 CrossRef CAS.
- F. Carnal and S. Stoll, J. Phys. Chem. A, 2012, 116, 6600–6608 CrossRef CAS PubMed.
- M. Le Bret and B. H. Zimm, Biopolymers, 1984, 23, 287–312 CrossRef CAS.
- T. G. Dewey, Biopolymers, 1990, 29, 1793–1799 CrossRef CAS.
- G. S. Manning and U. Mohanty, Physica A, 1998, 16, 196–204 Search PubMed.
- I. Borukhov, J. Polym. Sci., Part B: Polym. Phys., 2004, 42, 3598–3615 CrossRef CAS.
- A. Naji and R. R. Netz, Phys. Rev. Lett., 2005, 95, 185703 CrossRef PubMed.
- M. Muthukumar, J. Chem. Phys., 2004, 120, 9343–9350 CrossRef CAS PubMed.
- E. Raphael and J.-F. Joanny, Europhys. Lett., 1990, 13, 623–628 CrossRef CAS.
- R. Zhang and B. I. Shklovskii, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2005, 72, 021405 CrossRef PubMed.
- I. Sogami and N. Ise, J. Chem. Phys., 1984, 81, 6320–6332 CrossRef.
- J. G. Kirkwood and J. B. Shumaker, Proc. Natl. Acad. Sci. U. S. A., 1952, 38, 855–862 CrossRef CAS.
- D. Vlassopoulos, G. Fytas, T. Pakula and J. Roovers, J. Phys.: Condens. Matter, 2001, 13, R855–R876 CrossRef CAS.
- M. Kapnistos, M. Lang, D. Vlassopoulos, W. Pyckhout-Hintzen, D. Richter, D. Cho, T. Chang and M. Rubinstein, Nat. Mater., 2008, 7, 997–1002 CrossRef CAS PubMed.
- S. J. Dalsin, M. A. Hillmyer and F. S. Bates, ACS Macro Lett., 2014, 3, 423–427 CrossRef CAS.
- A. Chremos and J. F. Douglas, J. Chem. Phys., 2015, 143, 111104 CrossRef PubMed.
- M. E. Fox, Acc. Chem. Res., 2009, 42, 1141–1151 CrossRef CAS PubMed.
- A. Agrawal and S. Kulkarni, Int. J. Res. Dev. Pharm. Life Sci., 2015, 4, 1700–1712 Search PubMed.
- O. V. Borisov and E. Zhulina, Eur. Phys. J. B, 1998, 4, 205–217 CrossRef CAS.
- A. Jusufi, C. N. Likos and H. Löwen, Phys. Rev. Lett., 2001, 88, 018301 CrossRef PubMed.
- N. Hoffmann, C. N. Likos and H. Löwen, J. Chem. Phys., 2004, 121, 7009–7021 CrossRef CAS PubMed.
- M. Ballauff, Prog. Polym. Sci., 2007, 32, 1135–1151 CrossRef CAS.
- A. Jusufi and C. N. Likos, Rev. Mod. Phys., 2009, 81, 1753–1772 CrossRef.
- P. G. Dommersnes, Y. Kantor and M. Kardar, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2002, 66, 031802 CrossRef PubMed.
- R. Chang and A. Yethiraj, J. Chem. Phys., 2003, 118, 6634 CrossRef CAS.
- R. Chang and A. Yethiraj, Macromolecules, 2006, 39, 821–828 CrossRef CAS.
- J. Dudowicz, K. F. Freed and J. F. Douglas, J. Chem. Phys., 2015, 143, 131101 CrossRef PubMed.
- J. Dudowicz, K. F. Freed and J. F. Douglas, J. Chem. Phys., 2015, 143, 194901 CrossRef PubMed.
- A. Chremos and J. F. Douglas, MRS Advances, 2015 Search PubMed , submitted.
- K. Karatasos and M. Krystallis, J. Chem. Phys., 2009, 130, 114903 CrossRef CAS PubMed.
- A. Chremos and J. F. Douglas, J. Chem. Phys., 2015 Search PubMed , in preparation.
- G. S. Manning, Soft Matter, 2014, 10, 3738–3747 RSC.
- B. Halle and M. Davidovic, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 12135–12140 CrossRef CAS PubMed.
- L. Nilsson and B. Halle, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 13867–13872 CrossRef CAS PubMed.
- L. Zhang, L. Wang, Y.-T. Kao, W. Qui, Y. Yang, O. Okobiah and D. Zhong, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 18461–18466 CrossRef CAS PubMed.
- Although 1 ps would appear to be a “short” time, this period of time is longer than the time required for a sound wave to propagate an interatomic distances in a condensed fluid.77 This means that we may view the polymer and the associated interfacial ions and solvent as a single ‘rigid’ object from a hydrodynamic standpoint. Hydrodynamic measurement of proteins and other macromolecules made on picosecond and nanosecond timescales should reflect this collective unit in the decay in the self-intermediate scattering function, from which the diffusion coefficient can be estimated.78 This time scale is also long enough for inertia effects associated with fluid complessibility to become unimportant.79 Therefore, while a picosecond is a ‘short’ time by some standards, it is long enough to have important hydrodynamic consequences.
- I. Morfin, F. Horkay, P. J. Basser, F. Bley, A.-M. Hecht, C. Rochas and E. Geissler, Biophys. J., 2008, 87, 2897 CrossRef PubMed.
- V. M. Prabhu, E. J. Amis, D. P. Bossev and N. Rosov, J. Chem. Phys., 2004, 121, 4424 CrossRef CAS PubMed.
- V. M. Prabhu, B. D. Vogt, W. Wu, J. F. Douglas, E. K. Lin, S. K. Satija, D. L. Goldfarb and H. Ito, Langmuir, 2005, 21, 6647–6651 CrossRef CAS PubMed.
- T. E. Angelini, R. Golestanian, R. H. Coridan, J. C. Butler, A. Beraud, M. Krisch, H. Sinn, K. S. Schweizer and G. C. L. Wong, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 7962–7967 CrossRef CAS PubMed.
- M. Deserno, C. Holm and S. May, Macromolecules, 2000, 33, 199–206 CrossRef CAS.
- F. Bordi, C. Cametti and R. H. Colby, J. Phys.: Condens. Matter, 2004, 16, R1423–R1463 CrossRef CAS.
- K. Barros and E. Luijten, Phys. Rev. Lett., 2014, 113, 017801 CrossRef PubMed.
- K. S. Schmitz, Langmuir, 1997, 12, 5849–5863 CrossRef.
- N. Grønbench-Jensen, R. J. Mashl, R. F. Bruinsma and W. M. Gelbart, Phys. Rev. Lett., 1997, 78, 2477–2480 CrossRef.
- R. Messina, C. Holm and K. Kremer, Phys. Rev. Lett., 2000, 85, 872–875 CrossRef CAS PubMed.
- B. Hong, A. Chremos and A. Z. Panagiotopoulos, Faraday Discuss., 2012, 154, 29–40 RSC.
- J. Dudowicz, K. F. Freed and J. F. Douglas, J. Chem. Phys., 1999, 111, 7116–7130 CrossRef CAS.
- D. Stauffer and A. Aharony, Introduction to Percolation Theory, Taylor Francis, 1994 Search PubMed.
- G. Parisi and N. Sourlas, Phys. Rev. Lett., 1981, 46, 871–874 CrossRef CAS.
- F. Mallamace, E. Canetta, D. Lombaro, A. Mazzaglia, A. Romeo, L. M. Scolaro and G. Maino, J. Chem. Phys., 1969, 51, 924–933 CrossRef.
- R. Zwanzig and M. Bixon, Phys. Rev. A: At., Mol., Opt. Phys., 1970, 2, 2005–2012 CrossRef.
- F. Roosen-Runge, M. Henning, F. Zhang, R. M. J. Jacobs, M. Sztucki, H. Schober, T. Seydel and F. Schreiber, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 11815–11820 CrossRef CAS PubMed.
- R. Zwanzig and M. Bixon, J. Fluid Mech., 1975, 69, 21–25 CrossRef.
| This journal is © The Royal Society of Chemistry 2016 |