Thermo-responsive properties driven by hydrogen bonding in aqueous cationic gemini surfactant systems
Abstract
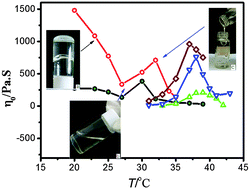
A series of unexpected thermo-responsive phenomena were discovered in an aqueous solution of the cationic gemini surfactant, 2-hydroxypropyl-1,3-bis(alkyldimethylammonium chloride) (n-3(OH)-n(2Cl), n = 14, 16), in the presence of an inorganic salt. The viscosity change trend for the 14-3(OH)-14(2Cl) system was investigated in the 20–40 °C temperature range. As the temperature increased, the viscosity of the solution first decreased to a minimum point corresponding to 27 °C, and then increased until a maximum was reached, after which the viscosity decreased again. In the 16-3(OH)-16(2Cl) system, the gelling temperature (Tgel) and viscosity changes upon heating were similar to those in the 14-3(OH)-14(2Cl) system above 27 °C. The reversible conversion of elastic hydrogel to wormlike micelles in the aqueous solution of the 16-3(OH)-16(2Cl) system in the presence of an inorganic salt was observed at relatively low temperatures. Various techniques were used to study and verify the phase-transition processes in these systems, including rheological measurements, cryogenic transmission electron microscopy (cryo-TEM), electric conductivity, and differential scanning calorimetry. The abovementioned phenomena were explained by the formation and destruction of intermolecular hydrogen bonds, and the transition mechanisms of the aggregates were analyzed accordingly.

 Please wait while we load your content...
Please wait while we load your content...