Uptake of phosphorus from surfactant solutions by wheat leaves: spreading kinetics, wetted area, and drying time
Courtney A. E.
Peirce
*a,
Craig
Priest
b,
Therese M.
McBeath
ac and
Mike J.
McLaughlin
ac
aThe University of Adelaide School of Agriculture, Food and Wine, Waite Campus, PB 1, Glen Osmond, SA 5064, Australia. E-mail: courtney.peirce@adelaide.edu.au
bIan Wark Research Institute, University of South Australia, Mawson Lakes, SA 5095, Australia
cCSIRO Agriculture Flagship, Waite Campus, PB 2, Glen Osmond, SA 5064, Australia
First published on 6th October 2015
Abstract
The delivery and uptake of nutrients at the surface of plant leaves is an important physicochemical phenomenon that depends on leaf surface morphology and chemistry, fertilizer formulation chemistry (including adjuvant and associated surfactants), wetting dynamics, and many other physical, chemical and biological factors. In this study, the role of spreading dynamics in determining uptake of the macronutrient phosphorus from phosphoric acid fertilizer solution in combination with three different adjuvants was measured in the absence of droplet run-off and splashing. When run-off and splashing losses were zero, spreading and drying rates had a small to negligible effect on the uptake efficiency. The results suggest that uptake may be much less sensitive to the specific choice of adjuvant and long time-scale spreading behaviour than one might intuitively expect.
Introduction
Wheat (Triticum aestivum) is an important cereal crop for human consumption and will play a major role in feeding the global population into the future. Worldwide production of wheat is expected to rise by 12% to 778 million tonnes by 2023, with USA and Australia among the leading exporters.1 The predicted growth in production over the next decade will require efficient fertilizer use to ensure adequate nutrient availability.2 Foliar fertilizers (application to the crop's leaves) offer a strategic approach to nutrient delivery through application during the growing season when climate conditions, which can influence the yield and growth of the crop, are known. In this paper, the static and dynamic wettability on the complex morphology of wheat leaves is compared with the uptake and translocation of foliar fertilizer (phosphoric acid).According to a recent study under controlled conditions, well-timed foliar delivery of phosphorus (P) fertilizer could boost wheat crops by up to 25% in good seasons.3 Phosphorus is essential for plant growth, yet it is often of low bioavailability in agricultural soils and must be applied as fertilizer to achieve viable crop yields. While considerable research has demonstrated the effectiveness of micronutrient foliar fertilizers4 and foliar nitrogen can be an effective management strategy,5 foliar applied P is still being explored for agricultural use.6 For a significant benefit, the successful delivery and uptake of the foliar fertilizer is vital and depends on both the specific surface properties of the leaf and the choice of fertilizer formulation.7
The surface morphology of plant leaves is extremely complex, often consisting of both micro and nano-scale surface structure and hydrophobic surface chemistry.8–13 Wheat leaves, for example, exhibit microscopic veins, stomata (openings for gas exchange), and trichomes (leaf hairs), as well as excreted nanocrystals of non-wetting epicuticular wax that cover the majority of the surface features (cf.Fig. 1).12 This combination of surface structure and chemistry renders wheat leaves difficult to wet with pure water,14 as observed for many other plant leaves (such as soybean,15 barley, wild oats16 and the well-known Lotus leaf13). The hydrophobicity of these leaves makes the addition of adjuvants that contain a surfactant to foliar sprays vital for their basic function and efficiency.7 An adjuvant is defined as any additive to a foliar spray that helps to modify the activity of the active ingredient (increase uptake) or modify the spray characteristics.17 Surfactants, one of the most common classes of adjuvants, lower the surface tension of the sprayed solution leading to a greater leaf–droplet contact area, adhesion and, ultimately, higher retention.
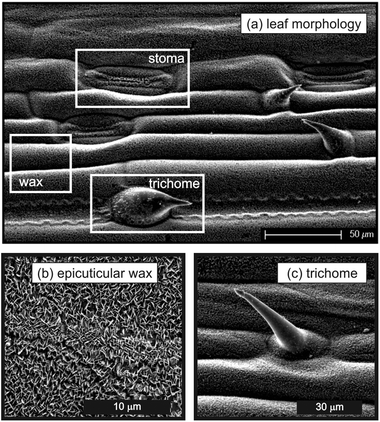 | ||
| Fig. 1 Scanning electron microscopy images of the adaxial (upper) side of a wheat leaf used in this study, showing the key features: trichome, stoma, and epicuticular wax. | ||
A review by Taylor18 highlights the many different factors involved in wetting of a leaf by an agrochemical solution. These factors include leaf surface composition, surface tension of the solution, environmental effects (such as weathering), adjuvant type, drop size, and others. Conventional wetting theories, such as the Cassie19 and Wenzel20 equations, are often inadequate for describing the wettability of leaves18 on which contact line pinning (and thus contact angle hysteresis) can dominate behaviour. This is also consistent with the results of wetting studies on a variety of highly structured synthetic surfaces.21–26 The physical picture is further complicated by wetting dynamics, which can play an important role during droplet–leaf impact with respect to splashing and run-off.16,27–30 Given the complexity of the mechanisms involved, it is a tempting convenience to assume that improved spreading (faster spreading to lower contact angles with increased leaf area in contact with the foliar spray) will increase the uptake of the fertilizer. This was tested experimentally in this study.
In this paper, spreading and evaporation (drying times) of foliar P solutions on wheat leaves was studied for three different fertilizer solutions and compared with results on flat hydrophobic plates. The chosen methodology prevents splashing, bouncing, run-off or other mechanical losses of the solution, ensuring that 100% of the P is available for uptake by the leaf for each solution. The early stage spreading showed general agreement with the power law dependence predicted by Tanner31 but showed late stage departure. Uptake and translocation of P via the foliar route was quantified and compared with the spreading and drying of the droplet. We show that the spreading rates and drying times studied are less important in determining the overall uptake efficiency than one might intuitively expect.
Experimental section
Plant growth and leaf morphology
Plants were grown in pots of 10 cm diameter and 17 cm depth that were not free-draining and held a total of 1.7 kg soil pot−1. The characteristics of the calcareous soil from South Australia used in this study are outlined in Peirce, et al.32 Briefly, the soil is an alkaline loam (pH 8.5) with a total P concentration of 48 mg kg−1 and classified as responsive to P fertilizer (Colwell-P 3 mg kg−1 and DGT-P 4 μg L−1).33 The soil was mixed with basal nutrients for plant growth at rates (based on pot surface area) equivalent to those outlined in Noack, et al.34 plus magnesium at 25 kg ha−1 as MgSO4·7H2O. Before sowing, the soil was watered to 70% field capacity (22.2% w/w) and P (7.2 kg P ha−1 as phosphoric acid) and nitrogen (N) (75 kg N ha−1 as urea) were banded 2 cm below the seed. At 24 days after sowing (DAS) an additional 25 kg N ha−1 as urea was applied in solution to the soil surface and watered in to ensure N was not limiting.Four pre-germinated seeds of wheat (Triticum aestivum cv. Axe) were sown in each pot and thinned to two with growth conditions as described in Peirce, et al.32 Plants were grown in a controlled environment room (20 °C/15 °C day/night cycle of 12 h each) and their positions randomised every week.
Microscopy
Wheat leaves (second leaf down on the main stem) were collected at flag leaf emergence (i.e., cereal growth stage Zadoks 3935) and small sections from the middle of the leaf avoiding the mid-rim were cut and fixed for scanning electron microscopy (SEM). The plant samples were fixed and vacuum infiltrated in 0.25% glutaraldehyde and 4.0% paraformaldehyde in phosphate buffer solution (PBS) with 4% sucrose at a pH of 7.2 overnight. Samples were then rinsed in PBS and 4% sucrose three times before post fixing in 2% osmium tetroxide in PBS for 1 h. They were then washed and progressively dehydrated in an ethanol series: 70% (2 changes of 15 min), 90% (2 changes of 15 min), and 100% ethanol (3 changes of 15 min) to remove water from internal cells and preserve cell structure. Samples were then critical point dried in a Bal-tec CPD 030 Critical Point Dryer, mounted on a stub and coated with 5 nm of platinum. Images were taken on a Philips XL20 scanning electron microscope under high vacuum at 10 kV and a working distance of 10 mm.Adjuvants
The three adjuvants analysed in this study were LI 700®, Agral® and Genapol® X-080. LI 700® is an acidifying and penetrating surfactant with 35% w/v propionic acid (CAS No. 79-09-4), 35% w/v soyal phospholipids (CAS No. 8002-43-5) and 10–30% w/v non-ionic surfactant. Agral® is a spray additive with 63% w/v nonyl phenol ethylene oxide condensate (CAS No. 9016-45-9). Genapol® X-080 is a pure surfactant of polyethylene glycol monoalkyl ether (CAS No. 9043-30-5). The equilibrium surface tension (beyond CMC) of LI 700® is 32 mN m−1 (CMC 1% w/w) and for Agral® is 34 mN m−1 (CMC 0.1% w/w).36 The surface tension of Genapol® X-080 at 0.1% is 27 mN m−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 (CMC 0.028% w/v).38
37 (CMC 0.028% w/v).38
Foliar uptake and translocation
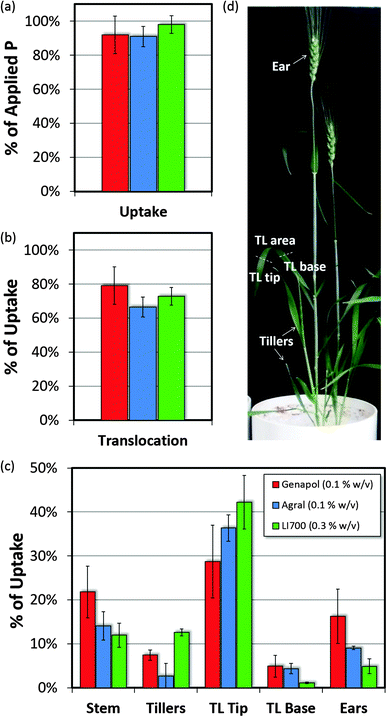
One concentration of each of the adjuvants in combination with phosphoric acid was chosen to assess foliar P uptake (fertilizer P recovered in all plant parts after washing as a percentage of foliar P fertilizer applied) and translocation of P (fertilizer P recovered in plant parts except the treated area after washing as a percentage of foliar P fertilizer uptake). The three foliar fertilizer treatments were applied to the leaves at mid booting (Zadoks 45, 36 DAS) replicated three times to give a total of nine pots. The rate of foliar applied P for all three treatments was 1.85% P w/v as orthophosphoric acid (Fisher Scientific Analytical Reagent 85% phosphoric acid CAS No. 7664-38-2) equivalent to 1.85 kg P ha−1 at a watering rate of 100 L ha−1 (approximately based on pot surface area). The three adjuvant concentrations used were 0.1% w/v (label rate) for Agral®, 0.1% w/v for Genapol® X-080, and 0.3% w/v (label rate) for LI 700®. Each foliar fertilizer was labelled with 33P in the orthophosphate form as a tracer to give a known specific activity for each treatment. The foliar fertilizer solutions were applied mid-morning to the second and third leaf down on the main stem with a micropipette in a dose split between the four leaves with five drops per leaf (approximately 4 μL drop−1).Above-ground plant parts were harvested seven days after foliar application. Plant parts were harvested 1 cm from above the soil base and divided into the following sections before washing: treated leaves, main stem and tillers. After washing, treated leaves were separated into the treated area, leaf tip and leaf base. Each of the plant parts was washed and analysed for total P and 33P activity as outlined in Fernández, et al.9
Analysis of variance (ANOVA) was undertaken to analyse differences in dry weights between treatments using Genstat® V.15 statistical package. Assumptions of distribution normality and constant error variance were tested. Least significant difference (l.s.d.) between treatments was determined at a significance level of p ≤ 0.05 using Fisher's Protected LSD.
Contact angle measurements
We measured the static advancing and receding contact angle of fertilizer solutions on the adaxial (upper) side of leaves from wheat plants at growth stages where field application of foliar fertilizer is feasible (Zadoks 40–50). Measurements were made using the sessile drop method (with the needle in the drop) and calculated based on observation of the profile of small water droplets (∼2 μL) (DataPhysics, OCAH 200) as described in Forsberg, et al.39 The initial droplet volume was brought into contact with the surface, increased slowly until the contact line advanced, and then stopped before a profile image of the droplet was captured and the contact angle measured. In the same way the liquid volume of the drop deposited onto the surface was decreased until the contact line receded, and then was allowed to relax before image capture of the droplet profile and contact angle measurement. There is a significant ridge in the middle of wheat leaves (mid-vein) which made contact angle measurements on an intact leaf difficult because the leaf does not lie flat. As a consequence, all contact angle measurements were made on an excised piece of the mid-section (length-wise) of the second leaf down on the main stem between the leaf edge and mid-vein immediately after detaching from the plant to ensure the leaf was not dried out. The excised leaf pieces were cut and stuck to glass slides with double-sided tape. Care was taken to avoid damage to the leaf surface: the leaf was only handled on its edges away from where contact angle measurements were made. As a result, a slight bump can sometimes been seen in the contact angle images (see Fig. 3) due to there being a small amount of air between the glass slide and leaf. The above procedure was critically important to ensure that measurements on different leaves and with different adjuvants were directly comparable.Before contact angles were measured for fertilizer solutions, the static advancing and receding contact angle for pure water (resistivity > 18 MΩ cm, Millipore) was measured on the leaf surface to ensure leaf surface wettability did not differ between these growth stages. The advancing contact angles of the fertilizer solutions (containing 2% P w/v and adjuvant at 0.01 to 0.3% w/v) were measured in the same way as the contact angle of water except that the droplets were allowed to relax for 20 s before images were taken. For fertilizer solutions on leaves, the receding contact angle could not be measured as the droplet was not observed to recede from the leaf surface, i.e., the receding contact angle was effectively 0° in every case. Contact angle values reported for fertilizers are the average of 20 measurements taken on two leaves from different plants and reported contact angle values for water are the average of 150 measurements taken over 30 leaf sections from 15 different plants.
Due to the differences in spreading dynamics observed for the different adjuvants, videos were taken of spreading for the three adjuvants. A 3 μL droplet was formed at the end of the needle and carefully brought into contact with the leaf surface before removing the needle. These videos were collected for a single concentration of each adjuvant which corresponded to the concentration used in the foliar uptake and translocation experiment; 0.1% w/v for Agral® and Genapol® X-080 and 0.3% w/v for LI 700®. Six replicates were taken for each adjuvant over six different leaves at early to mid-booting (Zadoks GS 40–45). From the video, single frames were extracted at selected time intervals for analysis using ImageJ and DropSnake40 software. When a contact angle of <10° was reached in two consecutive images, it was assumed that complete wetting (a film) had formed and so a nominal contact angle of 0° was recorded. This is standard practice in wettability studies and recognises the uncertainty in contact angle measurements below 10°. Likewise, a contact angle of 0° was recorded when a film escaping from the droplet was visually observed on the leaf surface (for Genapol® X-080 only), which indicates a ‘wicking’ event.21,41
Contact angles were also measured on flat hydrophobized glass plates for comparison with the leaves. Glass plates (1.1 mm thick, Borofloat 33, Schott) were cleaned in Piranha Solution (30% H2O2, 70% H2SO4). Caution: Piranha solution is strongly oxidising and reacts exothermically during preparation of the solution. The clean samples were then coated with octadecyltrichlorosilane (OTS) adsorbed from a 1% v/v OTS solution in toluene. The samples were immersed in the OTS solution for 30 min, then rinsed twice with toluene to remove excess OTS from the surface. The plates were then dried in a stream of high purity nitrogen before contact angle measurements.
Drying time and spreading area
The spread and drying times of fertilizer drops were observed on separate detached leaves immediately after detaching from the plant within the controlled environment room (20–22 °C, 38–40% RH). A 3 μL drop was placed on flat detached leaves (second leaf down on the main stem) with a micropipette. Using a stopwatch, the time until the drop had visually been absorbed/evaporated from the leaf i.e. no liquid was present on the leaf surface with the naked eye, was recorded for each drop. The drop spread was measured after the drop had dried by the scorch it produced on the leaves (which corresponded to where the drop had been placed). The spread area was then calculated using ImageJ. These measurements were once again only performed for a single concentration of each adjuvant which corresponded to the concentration used in the foliar uptake and translocation experiment; 0.1% w/v for Agral® and Genapol® X-080 and 0.3% w/v for LI 700®. A total of 10 replicates were taken for each adjuvant over 10 different leaves at early to mid-booting (Zadoks 40–45). Due to the hydrophobic nature of the leaves, 2% P without any adjuvant could not be measured on leaves because the drops would not detach from the pipette (the leaf was superhydrophobic).42Results and discussion
The adaxial (upper) side of wheat leaves had a complex surface structure with multiple levels of surface roughness as highlighted in Fig. 1. At the larger scale, there were ridges that ran parallel to the long axis of the leaf with stomatal pores forming dips regularly within the ridges. The individual cells of the leaf add further roughness along with the trichomes (leaf hairs) which were often observed near the stomata. At a smaller scale were waxes which covered the entire leaf surface including stomata and trichomes. The surface coverage of trichomes and stomata averaged 41–45 and 51–55 mm−2 respectively for the same cultivar of wheat grown under similar conditions previously.9,32These surface features are closely associated with the wettability of the leaf and the efficacy of sprayed crop protection chemicals and foliar fertilizers. In this study, the leaf structure was unchanged across all of our experiments, which was achieved by using plants that were exclusively within the wheat growth stages of early booting (Zadoks 40) and early ear emergence (Zadoks 50), whereas the applied fertilizer solutions were varied in adjuvant type and concentration. First, contact angles and spreading dynamics for the different adjuvants on wheat leaves were studied. Second, the correlation with P uptake, its transport within the plant (translocation), and the implications for the practical use of surface-acting additives in foliar P fertilizers are discussed.
Contact angle
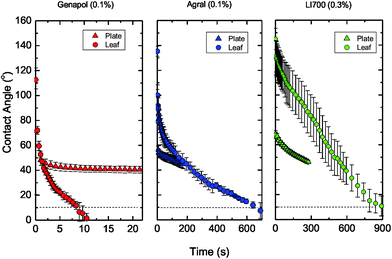
Advancing contact angles were measured 20 s after careful placement of a 2–3 μL droplet on the leaf for pure water and adjuvant concentrations up to 0.3% w/v (Fig. 2). The measurement time (20 s) was chosen to allow sufficient time to reach static wetting conditions; however, it was shown later that longer time-scales were necessary for two of the adjuvants.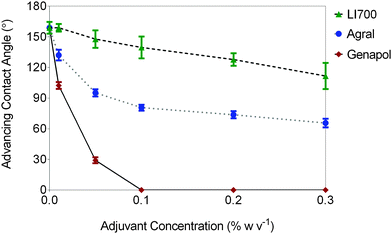 | ||
| Fig. 2 Advancing contact angle measured 20 s after deposition on wheat leaves for varying concentration of the adjuvants in 2% P w/v phosphoric acid (error bars represent standard deviation). | ||
First consider pure water. The static advancing and receding contact angles for water on the wheat leaves were 159 ± 6° and 149 ± 10°, respectively, and were time-independent after the initial relaxation of the droplet. Adding phosphoric acid (20 mg P mL−1) to the water did not affect the contact angle. These contact angles are consistent with previous measurements for water on wheat leaves.32 The very small amount of contact angle hysteresis implies that Cassie state wetting was being realised,42 where the droplet rested on top of the surface topography of the leaf. It is difficult to attribute the superhydrophobic behaviour to specific leaf surface features; however, the nanostructured ‘carpet’ of epicuticular wax (Fig. 1) is likely to have played a central role as for other superhydrophobic leaves.13 From these results, it is clear that for wheat, adjuvants which lower the surface tension of the solution and enhance spreading are very necessary in crop spray formulations to avoid the run-off of droplets due to superhydrophobicity.9
Three different surface-acting adjuvants were considered here. LI 700® and Agral® are commercially available and used for crop spraying. Genapol®X-080, is a powerful wetting agent of reagent grade (polyethylene glycol monoalkyl ether) which provided a comparison between the commercial adjuvants and a stronger surfactant. Contact angle measurements were carried out 20 s after droplet placement and were related to adjuvant concentration (Fig. 2). The advancing contact angle measured at 20 s decreased with increasing adjuvant concentration as expected. However, the receding contact angle (not shown) was zero (complete wetting) for each solution studied. A thin film of liquid remained on the leaf, even at the lowest adjuvant concentration when the contact angle was receded. It follows that very little adjuvant (<0.01% w/v) was required to prevent Cassie state wetting. For Genapol® X-080, the advancing contact angle reached complete wetting within 20 s for the 0.1% w/v solution. For the same concentration, the advancing contact angles were still very high for LI 700® (112°) and Agral® (81°). Our results qualitatively agree with the leaf coverage measurements for Agral® (at 0.1% v/v) and LI 700® (at 0.5% v/v) observed on field bean, pea and barley leaves by Holloway, et al.43 The contact angle for these two adjuvants decreased further with increasing adjuvant concentration until 0.3% w/v (the maximum concentration studied here). These short time-scale experiments already show a remarkable difference between the wetting behaviour induced by the three adjuvants.
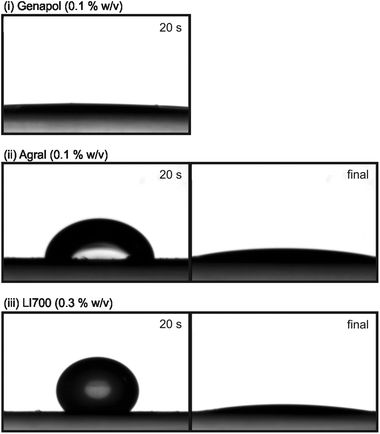
Fig. 3 shows images of the droplet profiles at 20 s and at the final contact angle after the droplet has finished spreading (20 min) for three different solutions (0.1% w/v Genapol® X-080, 0.1% w/v Agral®, and 0.3% w/v LI 700®). For Genapol® X-080 (0.1% w/v) the droplet had already reached complete wetting at 20 s so the second image is omitted. Given enough time, the contact angle decreased to less than 10° (which we regard as complete wetting) for each adjuvant solution. The large change in contact angle highlights the complexities present in studies of the collision–adhesion-spreading events at the droplet–leaf interface. For example, the three different solutions studied had the same final contact angle; however, in practice, faster dynamics of the droplet–leaf collision could reasonably render the final contact angle unimportant in determining the actual retention of the sprayed droplets. Furthermore, contact angle hysteresis, the low receding contact angle, and droplet size, amongst other factors, are important parameters for droplet retention. However, in this study, we have separated the collision dynamics from the spreading behaviour of the droplets. The droplet–leaf attachment was 100% successful due to careful droplet placement, so that run-off and splashing were excluded as loss mechanisms. In this way, the relationship between spreading dynamics, drying times, and uptake of P into the wheat leaves can be considered without complications arising from run-off.
Spreading dynamics
Despite our observation that each solution completely wetted the leaf after sufficient time (i.e. similar static wettability), the rate of spreading between fertilizer solutions varied greatly (Fig. 4). For Genapol® X-080 at 0.1% w/v, spreading was complete after only 10 s (Fig. 4). For Agral® (0.1% w/v) and LI 700® (0.3% w/v), spreading was complete after approximately 10 min and 15 min, respectively, i.e. more than 50 times slower than for Genapol® X-080 (0.1% w/v). In general, Genapol® X-080 and Agral® gave much more reproducible results than LI 700®, as indicated by the larger error bars for LI 700®.Consider a droplet with a radius less than the capillary length, 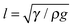 , where γ is the liquid–vapour surface tension, ρ is liquid density, and g is acceleration due to gravity, so that the effect of gravity on the drop shape is negligible. If the droplet completely wets the solid, the expansion of the base radius of the droplet, R, in time, t, can be predicted by Tanner's law, R(t) ∝ tn, where n is 0.1.31 The prediction n = 0.1 is generally in agreement with experiments.31,44–46 Exceptions include late time-scales (beyond the reasonable application of Tanner's law),47 solutions containing surfactants (including so-called ‘superspreaders’;48 very effective surfactants) that can increase n above 0.1,49–51 and some liquids, such as nematic liquid crystals.52–54 According to Tanner's law, plotting R versus t on a log–log plot should give a linear trend with slope n. In this study, direct measurements of R (in addition to contact angle, θ) were carried out to exclude errors due to evaporation (the base radius is related to the contact angle through the drop volume). Using the actual base radius and contact angle of the droplet at t = 1 s for Genapol® X-080 and t = 5 s for the slower spreading Agral® and LI 700® solutions, the droplet volume (pre-evaporation) could be calculated. These times were chosen to be early enough to negate evaporation and late enough to ensure that the spherical cap assumption was valid. The calculated droplet volume was 2.4 μL for Genapol® X-080 and 2.8 μL for Agral® and LI 700®, compared with the nominally dispensed volume of ∼3 μL. The difference is due to the detachment dynamics (necking) that occurs during placement, with faster spreading dynamics responsible for the larger discrepancy. The actual drop volumes were used to calculate the radius of the base of the droplet from the measured contact angles shown in Fig. 4. The results are plotted in Fig. 5 and compared with the measured radii at several different times (open symbols). The range of data used for fitting was decided based on including the maximum number of data points (from time zero) while ensuring that the coefficient of determination (R2) values were greater than 0.99 or, in the case of LI 700® where the fit was less good, the maximum R2 value obtainable. The close agreement between the two data sets shows that the acceleration of spreading at long time-scales was real (i.e. not an artifact of evaporation because of the effect of reducing the volume of a droplet with a pinned contact line). ‘Post-Tanner law’ departure (during late-stage spreading) has been discussed elsewhere by Mechkov54,55 and, although our experimental system is very different to that studied previously, the behavior appears to be similar. For earlier time-scales the results were linear and in good agreement with Tanner's power law. The linear fits for Agral® and LI 700® give n = 0.097 and 0.073, close to n = 0.1 as predicted by Tanner's law, although the quality of the fit for LI 700® (R2 = 0.94) was not as good as that for Agral® (R2 > 0.99). The best fit for the early timescale results of the faster spreading Genapol® X-080 solution gave a slightly elevated exponent, n = 0.159, which might be anticipated for some surfactants.48 Although, for Genapol® X-080, the departure from Tanner's law occurs within seconds of placement of the droplet on the leaf making the comparison with the theoretical prediction of Tanner quite limited. The power-law dependence observed was quite similar to selected experiments reported previously by Wang, et al.51 For example, the non-ionic trisiloxane surfactants used in their study gave n = 1/7 or 1/10 (10–1000 ms), compared with n = 0.159 (∼1/7) for Genapol (timescale 0.1–3 s) and n = 0.097 and 0.073 (∼1/10) for Agral and LI700 (timescale 0.5 to ∼100 s) in this study, respectively. However, for late stage spreading (timescale 1 to 10 s), Wang et al. reported n = 0.25 and 0.55 depending on the surfactant concentration. This dependence on surfactant type, concentration, and measurement timescale suggests that our results are not especially unusual with respect to the exponent values obtained.
, where γ is the liquid–vapour surface tension, ρ is liquid density, and g is acceleration due to gravity, so that the effect of gravity on the drop shape is negligible. If the droplet completely wets the solid, the expansion of the base radius of the droplet, R, in time, t, can be predicted by Tanner's law, R(t) ∝ tn, where n is 0.1.31 The prediction n = 0.1 is generally in agreement with experiments.31,44–46 Exceptions include late time-scales (beyond the reasonable application of Tanner's law),47 solutions containing surfactants (including so-called ‘superspreaders’;48 very effective surfactants) that can increase n above 0.1,49–51 and some liquids, such as nematic liquid crystals.52–54 According to Tanner's law, plotting R versus t on a log–log plot should give a linear trend with slope n. In this study, direct measurements of R (in addition to contact angle, θ) were carried out to exclude errors due to evaporation (the base radius is related to the contact angle through the drop volume). Using the actual base radius and contact angle of the droplet at t = 1 s for Genapol® X-080 and t = 5 s for the slower spreading Agral® and LI 700® solutions, the droplet volume (pre-evaporation) could be calculated. These times were chosen to be early enough to negate evaporation and late enough to ensure that the spherical cap assumption was valid. The calculated droplet volume was 2.4 μL for Genapol® X-080 and 2.8 μL for Agral® and LI 700®, compared with the nominally dispensed volume of ∼3 μL. The difference is due to the detachment dynamics (necking) that occurs during placement, with faster spreading dynamics responsible for the larger discrepancy. The actual drop volumes were used to calculate the radius of the base of the droplet from the measured contact angles shown in Fig. 4. The results are plotted in Fig. 5 and compared with the measured radii at several different times (open symbols). The range of data used for fitting was decided based on including the maximum number of data points (from time zero) while ensuring that the coefficient of determination (R2) values were greater than 0.99 or, in the case of LI 700® where the fit was less good, the maximum R2 value obtainable. The close agreement between the two data sets shows that the acceleration of spreading at long time-scales was real (i.e. not an artifact of evaporation because of the effect of reducing the volume of a droplet with a pinned contact line). ‘Post-Tanner law’ departure (during late-stage spreading) has been discussed elsewhere by Mechkov54,55 and, although our experimental system is very different to that studied previously, the behavior appears to be similar. For earlier time-scales the results were linear and in good agreement with Tanner's power law. The linear fits for Agral® and LI 700® give n = 0.097 and 0.073, close to n = 0.1 as predicted by Tanner's law, although the quality of the fit for LI 700® (R2 = 0.94) was not as good as that for Agral® (R2 > 0.99). The best fit for the early timescale results of the faster spreading Genapol® X-080 solution gave a slightly elevated exponent, n = 0.159, which might be anticipated for some surfactants.48 Although, for Genapol® X-080, the departure from Tanner's law occurs within seconds of placement of the droplet on the leaf making the comparison with the theoretical prediction of Tanner quite limited. The power-law dependence observed was quite similar to selected experiments reported previously by Wang, et al.51 For example, the non-ionic trisiloxane surfactants used in their study gave n = 1/7 or 1/10 (10–1000 ms), compared with n = 0.159 (∼1/7) for Genapol (timescale 0.1–3 s) and n = 0.097 and 0.073 (∼1/10) for Agral and LI700 (timescale 0.5 to ∼100 s) in this study, respectively. However, for late stage spreading (timescale 1 to 10 s), Wang et al. reported n = 0.25 and 0.55 depending on the surfactant concentration. This dependence on surfactant type, concentration, and measurement timescale suggests that our results are not especially unusual with respect to the exponent values obtained.
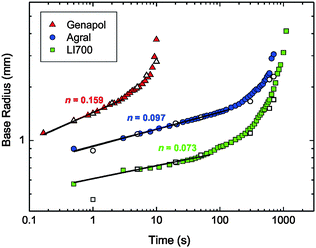 | ||
| Fig. 5 Calculated droplet base radius (coloured symbols) using the results presented in Fig. 4 (see discussion) and plotted against time on a log–log plot. Direct measurements of radius for selected times are shown for comparison (white symbols). Linear fits according to Tanner's law are shown for short time-scales only (see discussion). | ||
Spreading occurred over tens of minutes for Agral® and LI 700® and therefore evaporation should not be completely ignored. Despite complete wetting being observed for each solution, the final surface area covered for the Genapol® X-080 solution was three times greater than that for the Agral® and LI 700® solutions (Table 1). The time to drying, also included in Table 1, was in qualitative agreement with the spread area results, i.e. larger spread areas led to shorter drying times. The Genapol® X-080 solution dried fastest (16 min), while the drying times for Agral® and LI 700® were almost identical at ∼35 min. These drying times were determined by visual observations and it is important to note that the uptake of foliar fertilizers into leaves and fertilizer drying occurs concurrently. Also, evaporation of water from the droplet is increasing the adjuvant (surfactant) concentration and, thus, the rate of spreading. However, the departure from Tanner's law cannot be attributed to evaporation because the departure (acceleration) in the spreading of the Genapol® X-080 solution was observed after only a few seconds, which is well before evaporation could be considered significant. For Genapol® X-080, the time-to-drying was more than 100 times greater than the elapsed time prior to departure from linearity (several seconds). For Agral® and LI 700®, the time-to-drying was ∼20 times longer. Therefore, it appears unlikely that evaporation was the primary driver for the accelerated spreading observed in Fig. 5.
| Solution | Time to drying (s) | Spread (mm2) |
|---|---|---|
| Genapol® 0.1% w/v | 956 ± 165 (16 min) | 68 ± 11 |
| Agral® 0.1% w/v | 2088 ± 195 (35 min) | 23 ± 4 |
| LI 700® 0.3% w/v | 2242 ± 480 (37 min) | 22 ± 5 |
Surface roughness is another parameter that should be considered. For comparison, flat glass plates were hydrophobized using octadecyltrichlorosilane (see Experimental section) to mimic the wettability of the aliphatic epicuticular wax chemistry (1-octacosanol for wheat12) without introducing additional roughness. Spreading results for the three solutions on this model surface generally gave less uncertainty compared to our measurements on the leaves (Fig. 4). In addition, the final contact angle was much higher in each case, typically close to 40°. The effect of roughness on the contact angle is often described using a roughness factor, r, introduced by Wenzel:20 cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θr = r·cos
θr = r·cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, where θr and θ are contact angles on the rough and ideal (flat) surfaces of the same material, and r is defined as the ratio between the actual and projected solid surface areas. For θ > 90°, roughness is predicted to increase θr, and, for θ < 90°, decrease θr. Wenzel's equation must be carefully applied, given it cannot account for contact line pinning (contact angle hysteresis) which is ubiquitous in wettability measurements. This point of caution is consistent with a recent review on leaf wetting,18 which refers to the importance of considering contact angle hysteresis in the context of run-off and practical limitations to the use of Wenzel's equation. Nonetheless, the predicted effect of enhanced wetting for θ < 90° holds in the present study. For θ0 = 40°, complete spreading (θr = 0) is predicted when the roughness factor, r, is greater than 1.3. The latter condition is reasonable given the highly structured leaf surfaces studied.
θ, where θr and θ are contact angles on the rough and ideal (flat) surfaces of the same material, and r is defined as the ratio between the actual and projected solid surface areas. For θ > 90°, roughness is predicted to increase θr, and, for θ < 90°, decrease θr. Wenzel's equation must be carefully applied, given it cannot account for contact line pinning (contact angle hysteresis) which is ubiquitous in wettability measurements. This point of caution is consistent with a recent review on leaf wetting,18 which refers to the importance of considering contact angle hysteresis in the context of run-off and practical limitations to the use of Wenzel's equation. Nonetheless, the predicted effect of enhanced wetting for θ < 90° holds in the present study. For θ0 = 40°, complete spreading (θr = 0) is predicted when the roughness factor, r, is greater than 1.3. The latter condition is reasonable given the highly structured leaf surfaces studied.
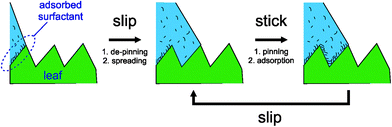
Roughness can enhance wetting (see above) or introduce pinning if the material contact angle is high. A special case of enhanced wetting is ‘wicking’ where a liquid film develops at the three-phase contact line and grows within the surface structure by capillarity. Wicking can be predicted thermodynamically based on the specific surface geometry and the material contact angle41 but is also opposed by pinning effects.21 For highly structured hydrophobic surfaces, pinning effects can dominate behaviour, such as on pillar arrays and similar surfaces.21–26 It follows that a surface with a transient wettability, e.g. due to surfactant adsorption from the adjuvant solution, will induce stick–slip behaviour,56,57 where the contact line is temporarily pinned until a condition for de-pinning is reached. It is therefore very likely that stick–slip behaviour is behind the observations in this study. In a simple model, surfactant adsorption takes place predominantly during the ‘stick’ period, while the contact line is pinned. The contact line will remain pinned on a surface asperity until sufficient surfactant is adsorbed on the solid–liquid interface to de-pin the liquid. Once de-pinned, the contact line will advance rapidly over fresh solid surface beyond the asperity (the ‘slip’ moment) and continue to spread until it re-pins on a subsequent surface asperity (as illustrated in Fig. 6). It follows that the ‘stick’ period must be longer for slower adsorbing or less effective surfactants, retarding the average velocity of the contact line. In practice, the stick–slip phenomena results in slower average spreading velocities on the rough surface (the wheat leaves) compared with flat surfaces of similar material contact angle (flat plates), which is clearly evident in Fig. 4 for Agral® and LI 700® solutions. For Genapol® X-080, the inherently fast spreading behaviour on the flat surface translated to a brief retardation of the spreading rate on the rough surface, i.e. within the first 1–2 s, while the overall time taken to reach the final contact angle was similar on flat and rough surfaces (∼10 s).
Thus far, we have only considered the spreading rate, wetted area, and time-to-drying. From the complexity of this spreading behaviour – particularly the different time-scales involved – one might intuitively expect that the total uptake (absorption) of the nutrient (P) into the leaf will vary for the different fertilizer/adjuvant solutions. In the following section, we report foliar uptake and translocation of P into wheat leaves and consider whether there is any correlation with spreading/drying rates.
Uptake and translocation
For P uptake and translocation experiments on wheat leaves, the industry recommended concentrations of adjuvants for crop spraying (label rates) were used: 0.3% w/v LI 700® and 0.1% w/v Agral®. For Genapol® X-080, the concentration was chosen to yield complete wetting within 20 s; 0.1% w/v. The droplet placement technique avoided run-off (see Experimental section), so that the results presented here are exclusively related to the physiological uptake, spreading kinetics, and drying times. The above ground dry weights of plants for the three different adjuvant treatments differed (p ≤ 0.05) (Table 2), with the Agral® treated plants being the smallest. This related mainly to differences in the weight of the main stem (which included the treated leaves) with both ear and tiller weights showing high variability between replicates across all treatments. Despite there being no statistical difference in tiller dry weight between treatments, the difference in total number of leaves can be attributed to tillers, averaging 12, 8 and 14 for the Genapol® X-080, Agral® and LI 700® treatments respectively. High heterogeneity in tillering as experienced in the experiment has also been shown by Rodriguez, et al.58 in response to low P availability. Since the number of leaves from the main stem did not differ between treatments, the lower plant weights for Agral® are likely to be due to reduced stem elongation of the plants due to the plants progressing slower through their growth stages. This delay in plant growth, delay and decrease in tiller emergence and lower dry weight of ears are all responses to P deficiency in wheat.58,59| Solution | Dry weight (mg) | |||
|---|---|---|---|---|
| Main stem | Tillers | Ear | Total plant (no. of leaves) | |
| Genapol® | 1308b | 489 | 415 | 2212b (21) |
| Agral® | 1041a | 207 | 351 | 1598a (17) |
| LI 700® | 1362b | 623 | 386 | 2371b (23) |
| l.s.d. (p ≤ 0.05) | 82 | n.s. | n.s. | 484 |
Phosphorus measured in the plant consists of P taken up from the soil, from the seed and from the P applied to the leaves (the foliar fertilizer). Although the P content in the tillers of the Agral® plants was significantly lower than LI 700® plants (0.9, 2.5 and 3.7 mg pot−1 for Agral®, Genapol® X-080 and LI 700® respectively, l.s.d. 2.1 p ≤ 0.05), the total plant P content did not differ between treatments and was 9.5 ± 2.6 mg pot−1 with 1.4 ± 0.1 mg taken up from the foliar-applied fertilizer. This foliar P contributed almost 15% of the total P in the plant but only 0.46 ± 0.17 mg had translocated to other plant parts (main stem, tillers and ears) and was located outside the treated leaves. The foliar uptake averaged 94% of what was applied and was not different between treatments; see Fig. 7(a). Of the remaining 6% of foliar P, 2% was washed from the surface of the leaves with a further 4% not recovered. Since there was no visible loss of foliar fertilizer at application, this unrecovered P is likely to be located in the roots. Thus, foliar uptake may be as high as 98% in this experiment. The high recovery of foliar fertilizer in above ground plant parts is in good agreement with previous studies on foliar P using phosphoric acid and LI 700®.3,32 However to our knowledge this is the first comparative study measuring uptake of foliar P fertilizer combined with different adjuvants using radiotracer techniques on in situ plants.
The amount of foliar P that translocated out of the treated leaf area (to all other plant parts including the treated leaf tip and base) was not significantly different between treatments averaging 73% of the absorbed foliar P (Fig. 7). More foliar P was translocated out of the treated leaf as a whole (i.e. to the main stem, tillers and ears) for the Genapol® X-080 treatment than the Agral® or LI 700® treatments. The sinks for foliar P were also different with LI 700® treated plants sending more foliar P to the tillers (13%) than both Genapol® X-080 (7%) and Agral® (3%) but less to the ears (5%) than Genapol® X-080 (16%).
It was shown by Marshall and Wardlaw60 that the movement of foliar-applied 32P is mainly determined by the movement and demand for carbohydrates rather than the P requirement. They also found that within 24 h there was 90% uptake of the 32P but most of it remained within the application site. Movement of 32P to the tip of the treated leaf occurred via the xylem (with transpiration and water flow) but other translocation occurred via the phloem to wherever the plant required carbohydrate (i.e. at locations of plant growth). In our study, it appears that more foliar P was translocated in the phloem for the Genapol® X-080 treatment than the Agral® and LI 700® treatments. The P that travelled in the xylem to the treated leaf tips may be ineffectual uptake if the scorch from the foliar applied phosphoric acid causes necrosis of the leaf cells. This would prevent the foliar applied P that had translocated to the tips from transporting to the rest of the plant and it would therefore be unlikely to contribute to the P nutrition of the plant.
Spreading and uptake
The overall foliar uptake of P from each of the fertilizer solutions was as high as 98% of the P applied and statistically identical for the three different adjuvants. For foliar fertilizers to be available for uptake, it is widely acknowledged that the fertilizer must be in solution,7 and therefore dependent on the time to drying. In our experiments, drying times varied from 16 to 37 minutes (Table 1) with harvest 7 days later. The longer time between application and harvest was to allow for translocation to occur but is not expected to have played a significant role in the uptake due to the drying time being much shorter than the length of time between application and harvest. Although faster drying is likely to decrease the available time for uptake, the fast-drying Genapol® X-080 solution also covered over three-times larger leaf area compared with the other two adjuvants, permitting a higher overall surface area for uptake that likely compensated for the shorter time before drying. These results suggest that each adjuvant was equally effective in achieving uptake, despite the different spreading kinetics and drying times observed. With respect to total uptake of P, the chemical nature of the adjuvants appears to be unimportant when applied in combination with phosphoric acid. This finding was unexpected and has important implications for foliar fertilizer formulation. In particular, our results indicate that the use of wettability (contact angles and spreading kinetics) to help rank the efficacy of adjuvants for use in foliar fertilizers should be carried out with caution, particularly when short time-scale wetting measurements are employed. In addition, the time-to-drying observed here (tens of minutes) appears to be more than sufficient to ensure maximum uptake of the P from the foliar fertilizer.Conclusions
We have studied the relationship between wetting behaviour and the efficacy of foliar P fertilizer solutions on wheat leaves in the absence of splashing and run-off. At short time-scales, the spreading behaviour could be described by Tanner's law; however, longer time-scales resulted in a significant departure (acceleration of the contact line) for the adjuvant solutions studied. It was shown that the rates of spreading and drying do not have a significant impact on the uptake of P into the leaf for phosphoric acid applied in combination with three different adjuvants. This was despite the spreading time-scale differing by more than two orders of magnitude, the drying times varying from 16 to 37 min, and the wetted leaf area ranging from 22 to 68 mm2. The results presented here illustrate the importance of the timescales of the physical phenomena involved, such as droplet attachment, spreading, drying, uptake into the leaf, and transport within the plant. We propose that successful retention of the solution at the leaf interface (collision and attachment) is the dominant factor in uptake efficiency and that wetting of large leaf areas and long drying times play only a minor role for the system studied. Examination of other fertilizer/adjuvant formulations is needed to yield the insight necessary for successful application of P and other nutrients via crop sprays.Acknowledgements
The authors thank the Grains Research and Development Corporation, The Commonwealth Hill Trust and the Fluid Fertilizer Foundation for financial support. Thanks to Rossen Sedev for helpful discussions and Bogumila Tomczak, Colin Rivers, Anil Palamattath, and Pontus Forsberg for technical assistance. All microscopy work was undertaken at the Adelaide Microscopy Waite Facility with technical assistance from Gwen Mayo. This work was performed in part at the South Australian node of the Australian National Fabrication Facility, a company established under the National Collaborative Research Infrastructure Strategy to provide nano and micro-fabrication facilities for Australia's researchers.References
- OECD/FAO, OECD-FAO Agricultural Outlook 2014, OECD Publishing, Paris, 2014.
- P. Marschner and Z. Rengel, in Marschner's Mineral Nutrition of Higher Plants, ed. P. Marschner, Academic Press, San Diego, 3rd edn, 2012, pp. 315–330 Search PubMed.
- T. M. McBeath, M. J. McLaughlin and S. R. Noack, Crop Pasture Sci., 2011, 62, 58–65 CrossRef.
- V. Fernández, T. Sotiropoulos and P. Brown, Foliar Fertilization: Scientific Principles and Field Practices, International Fertilizer Industry Association, Paris, France, 2013 Search PubMed.
- M. J. Gooding and W. P. Davies, Nutr. Cycling Agroecosyst., 1992, 32, 209–222 CAS.
- S. R. Noack, T. M. McBeath and M. J. McLaughlin, Crop Pasture Sci., 2011, 62, 659–669 CrossRef.
- V. Fernández and T. Eichert, Crit. Rev. Plant Sci., 2009, 28, 36–68 CrossRef.
- P. J. Holloway, Pestic. Sci., 1970, 1, 156–163 CrossRef CAS.
- V. Fernández, P. Guzmán, C. A. E. Peirce, T. M. McBeath, M. Khayet and M. J. McLaughlin, Plant Soil, 2014, 384, 7–20 CrossRef.
- K. Koch and H. J. Ensikat, Micron, 2008, 39, 759–772 CrossRef CAS PubMed.
- K. Koch, B. Bhushan and W. Barthlott, Soft Matter, 2008, 4, 1943–1963 RSC.
- K. Koch, W. Barthlott, S. Koch, A. Hommes, K. Wandelt, W. Mamdouh, S. De-Feyter and P. Broekmann, Planta, 2006, 223, 258–270 CrossRef CAS PubMed.
- W. Barthlott and C. Neinhuis, Planta, 1997, 202, 1–8 CrossRef CAS.
- H. de Ruiter, A. J. M. Uffing, E. Meinen and A. Prins, Weed Sci., 1990, 38, 567–572 CAS.
- D. W. M. Puente and P. Baur, Pest Manage. Sci., 2011, 67, 798–806 CrossRef CAS PubMed.
- J. R. Lake, Pestic. Sci., 1977, 8, 515–520 CrossRef.
- G. R. Stephenson, I. G. Ferris, P. T. Holland and M. Nordberg, Pure Appl. Chem., 2006, 78, 2075–2154 CrossRef CAS.
- P. Taylor, Curr. Opin. Colloid Interface Sci., 2011, 16, 326–334 CrossRef CAS.
- A. B. D. Cassie and S. Baxter, Trans. Faraday Soc., 1944, 40, 547–551 RSC.
- R. N. Wenzel, Ind. Eng. Chem., 1936, 28, 988 CrossRef CAS.
- C. Semprebon, P. Forsberg, C. Priest and M. Brinkmann, Soft Matter, 2014, 10, 5739–5748 RSC.
- M. Kanungo, S. Mettu, K.-Y. Law and S. Daniel, Langmuir, 2014, 30, 7358–7368 CrossRef CAS PubMed.
- D. M. Spori, T. Drobek, S. Zürcher and N. D. Spencer, Langmuir, 2010, 26, 9465–9473 CrossRef CAS PubMed.
- D. M. Spori, T. Drobek, S. Zürcher, M. Ochsner, C. Sprecher, A. Mühlebach and N. D. Spencer, Langmuir, 2008, 24, 5411–5417 CrossRef CAS PubMed.
- C. Priest, T. W. J. Albrecht, R. Sedev and J. Ralston, Langmuir, 2009, 25, 5655 CrossRef CAS PubMed.
- C. Dorrer and J. Rühe, Langmuir, 2007, 24, 1959 CrossRef PubMed.
- W. Wirth, S. Storp and W. Jacobsen, Pestic. Sci., 1991, 33, 411–420 CrossRef CAS.
- J. A. Zabkiewicz, Crop Prot., 2007, 26, 312–319 CrossRef.
- A. Fritsch, G. R. Willmott and M. Taylor, J. R. Soc. N. Z., 2013, 43, 198–210 CrossRef.
- L. Chen, Z. Xiao, P. C. H. Chan, Y.-K. Lee and Z. Li, Appl. Surf. Sci., 2011, 257, 8857–8863 CrossRef CAS.
- L. H. Tanner, J. Phys. D: Appl. Phys., 1979, 12, 1473 CrossRef CAS.
- C. A. E. Peirce, T. M. McBeath, V. Fernández and M. J. McLaughlin, Plant Soil, 2014, 384, 37–51 CrossRef CAS.
- T. M. McBeath, M. J. McLaughlin, R. D. Armstrong, M. Bell, M. D. A. Bolland, M. K. Conyers, R. E. Holloway and S. D. Mason, Aust. J. Soil Res., 2007, 45, 448–458 CrossRef CAS.
- S. R. Noack, M. J. McLaughlin, R. J. Smernik, T. M. McBeath and R. D. Armstrong, Plant Soil, 2014, 378, 125–137 CrossRef CAS.
- J. C. Zadoks, T. T. Chang and C. F. Konzak, Weed Res., 1974, 14, 415–421 CrossRef.
- M. Aytouna, D. Bartolo, G. Wegdam, D. Bonn and S. Rafaï, Exp. Fluids, 2010, 48, 49–57 CrossRef CAS.
- M. Khayet and V. Fernandez, Theor. Biol. Med. Modell., 2012, 9, 45 CrossRef CAS PubMed.
- S. R. Sirimanne, D. G. Patterson Jr, L. Ma and J. B. Justice Jr, J. Chromatogr. B: Biomed. Sci. Appl., 1998, 716, 129–137 CrossRef CAS.
- P. S. H. Forsberg, C. Priest, M. Brinkmann, R. Sedev and J. Ralston, Langmuir, 2010, 26, 860–865 CrossRef CAS PubMed.
- A. F. Stalder, G. Kulik, D. Sage, L. Barbieri and P. Hoffmann, Colloids Surf., A, 2006, 286, 92–103 CrossRef CAS.
- J. Bico, U. Thiele and D. Quéré, Colloids Surf., A, 2002, 206, 41–46 CrossRef CAS.
- A. Lafuma and D. Quere, Nat. Mater., 2003, 2, 457–460 CrossRef CAS PubMed.
- P. J. Holloway, M. C. Butler Ellis, D. A. Webb, N. M. Western, C. R. Tuck, A. L. Hayes and P. C. H. Miller, Crop Prot., 2000, 19, 27–37 CrossRef CAS.
- A. M. Cazabat and M. A. Cohen Stuart, J. Phys. Chem., 1986, 90, 5845 CrossRef CAS.
- P. Levinson, A. M. Cazabat, M. A. Cohen Stuart, F. Heslot and S. Nicolet, Rev. Phys. Appl., 1988, 23, 1009–1016 CrossRef.
- D. Ausserré, A. M. Picard and L. Léger, Phys. Rev. Lett., 1986, 57, 2671–2674 CrossRef PubMed.
- S. L. Cormier, J. D. McGraw, T. Salez, E. Raphaël and K. Dalnoki-Veress, Phys. Rev. Lett., 2012, 109, 154501 CrossRef PubMed.
- S. Rafaï, D. Sarker, V. Bergeron, J. Meunier and D. Bonn, Langmuir, 2002, 18, 10486–10488 CrossRef.
- T. Stoebe, Z. Lin, R. M. Hill, M. D. Ward and H. T. Davis, Langmuir, 1997, 13, 7270–7275 CrossRef CAS.
- T. Stoebe, R. M. Hill, M. D. Ward and H. T. Davis, Langmuir, 1997, 13, 7276–7281 CrossRef CAS.
- X. Wang, L. Chen, E. Bonaccurso and J. Venzmer, Langmuir, 2013, 29, 14855–14864 CrossRef CAS PubMed.
- C. Poulard, M. Voué, J. De Coninck and A. M. Cazabat, Colloids Surf., A, 2006, 282–283, 240–246 CrossRef.
- C. Poulard and A. M. Cazabat, Langmuir, 2005, 21, 6270–6276 CrossRef CAS PubMed.
- S. Mechkov, A. M. Cazabat and G. Oshanin, J. Phys.: Condens. Matter, 2009, 21, 464134 CrossRef CAS PubMed.
- S. Mechkov, A. M. Cazabat and G. Oshanin, J. Phys.: Condens. Matter, 2009, 21, 464131 CrossRef CAS PubMed.
- P. G. de Gennes, EPL, 1997, 39, 407 CrossRef CAS.
- M. E. R. Shanahan, Langmuir, 1995, 11, 1041–1043 CrossRef CAS.
- D. Rodriguez, F. H. Andrade and J. Goudriaan, Plant Soil, 1999, 209, 283–295 CrossRef CAS.
- D. Rodriguez, M. C. Pomar and J. Goudriaan, Plant Soil, 1998, 202, 149–157 CrossRef CAS.
- C. Marshall and I. Wardlaw, Aust. J. Biol. Sci., 1973, 26, 1–14 CAS.
| This journal is © The Royal Society of Chemistry 2016 |